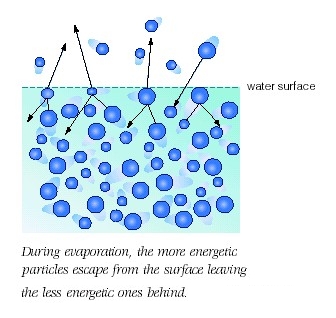
What is needed for evaporation to occur? Explain in terms of intermolecular forces.
1 Answer
In order to evaporate, the water molecules must overcome their intermolecular forces of attraction.
Explanation:
In order for water to evaporate, its molecules must have enough kinetic energy to allow them to overcome the relatively strong intermolecular forces (IMFs) of attraction that keeps the molecule together in the liquid phase.
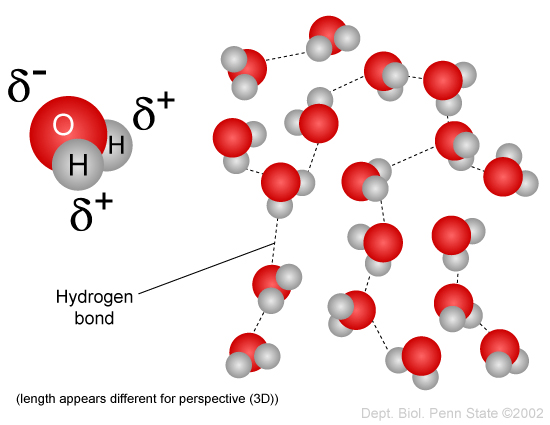
In liquid phase, water molecules are bonded together through hydrogen bonds that are constantly being broken and reformed as molecules collide with each other.
It is worth noting though, that a hydrogen-bond IMF is not an actual chemical bond. It is a (usually) short-range interaction.
When these molecules acquire sufficient kinetic energy, they manage to escape liquid phase and move into gas phase, where the intermolecular forces of attraction that existed between them are assumed to be negligible.
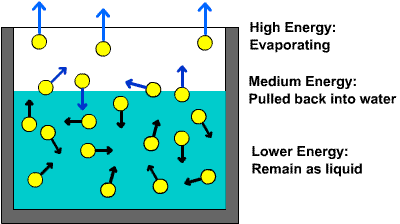
This is essentially what happens when you heat liquid water. As you provide more and more energy, an increasing number of water molecules will manage to break from the surface of the liquid.
In order for that to happen, the kinetic energy of the molecules must overcome the intermolecular forces of attraction.