Question #9e5d6
1 Answer
That depends on how much methane you're dealing with.
Explanation:
The answer to your question depends on whether or not you meant in a molecule of methane or in a mole of methane (or in some specific mass of methane).
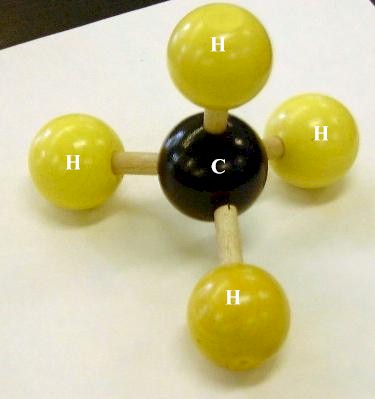
A methane molecule,
- one carbon atom
- four hydrogen atoms
arranged in a tetrahedral molecular geometry.
To get the number of atoms in a mole of methane, you need to use the definition of a mole.
In your case, a mole of methane represents a collection of methane molecules. More specifically, a mole of methane contains
Basically, to have one mole of methane, you need to have a total of
#underbrace(6.022 * 10^(23))_(color(blue)("1 mole CH"_4))color(red)(cancel(color(black)("molecules CH"""_4))) * "5 atoms"/(1color(red)(cancel(color(black)("molecule CH"""_4)))) = 3.01 * 10^(24)"atoms"#
What if you have a certain mass of methane? Let's say that you need to find how many atoms you have in
Since you already know how to use moles and molecules, you need to find a way to convert the mass of methane into moles of methane.
To do that, use methane's molar mass, which tells you exactly what the mass of one mole of methane is.
Methane's molar mass is
#25.0color(red)(cancel(color(black)("g CH"""_4))) * ("1 mole CH"""_4)/(16.042color(red)(cancel(color(black)("g CH"""_4)))) = "1.56 moles CH"""_4#
How many molecules of methane you have in this many moles? Use Avogadro's number again
#1.56color(red)(cancel(color(black)("moles CH"""_4))) * (6.022 * 10^(23)"molec. CH"""_4)/(1color(red)(cancel(color(black)("mole CH"""_4)))) = 9.39 * 10^(23)"molec CH"""_4#
Finally, this number of molecules will contain
#underbrace(9.39 * 10^(23))_(color(blue)("1.56 moles CH"_4))color(red)(cancel(color(black)("molec. CH"""_4))) * "5 atoms"/(1color(red)(cancel(color(black)("molec. CH"""_4)))) = 4.70 * 10^(24)"atoms"#

