How do you identify stereocenters on a complex molecule?
1 Answer
Perhaps you can analogize off of the answer I will put here. This is a rather comprehensive molecule I got on an exam for identifying stereocenters:
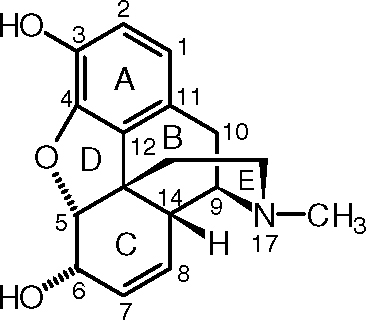
Morphine
- Carbon-5, connected to an
#1^o# alkoxide group, a tert-butyl group, a proton, and a#2^o# alcohol group. The priorities from high to low are:#1^o# alkoxide,#2^o# alcohol, tert-butyl, proton
Since the proton is in the front, and you go counterclockwise to follow these priorities, you get the reverse of S, which is R.
- Carbon-6, connected to a
#2^o# alkoxide group, a vinyl or alkenyl group, a proton, and a hydroxyl group. The priorities from high to low are:hydroxyl,
#2^o# alkoxide, vinyl (counted similar to isopropyl), proton
Since the proton is in the front, and you go clockwise to follow these priorities, you get the reverse of R, which is S.
- Carbon-9, connected to a
#2^o# amine group, a proton, a methylene group (#CH_2# ), and an isopropyl group. The priorities from high to low are:#2^o# amine, isopropyl, methylene, proton
Since the proton is in the back and you go clockwise to follow the priorities, this is R.
- Carbon-13 (the one that branches upwards), connected to a benzene ring, a
#2^o# alkoxide group, an isopropyl group, and a#1^o# alkyl group. The priorities from high to low are:#2^o# alkoxide, benzene ring (counted similar to tert-butyl), isopropyl,#1^o# alkyl
Since the
- Carbon-14, connected to a proton, a tert-butyl group, a vinyl or alkenyl group, and a
#2^o# amine group. The priorities from high to low are:#2^o# amine, tert-butyl, vinyl (counted similar to isopropyl), proton
Since the proton is in the front and you go counterclockwise to follow the priorities, this is the reverse of S, which is R.

