Question #81fd8
1 Answer
It would carry a
Explanation:
Beryllium,
This means that a neutral beryllium atom will have a total of 4 electrons surrounding its nucleus.
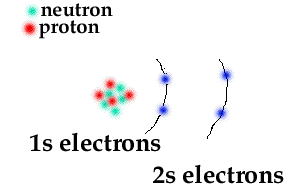
Now, out of these four electrons,
#"Be": ["He"] 2s^2#
So, if beryllium were to give up its valence electrons, what charge would you expect the resulting cation to have?
Well, since the atom would have to give up two electrons, both from the 2s-orbital, the charge of the cation would be
#"Be" -> "Be"^(+) + 1e^(-)#
The electron configuration of the
#"Be"^(+): ["He"] 2s^1#
and
#"Be"^(+) -> "Be"^(2+) + 1e^(-)#
with the electron configuration of the cation being
#"Be"^(2+): ["He"]#
Now, beryllium doesn't actually form cations because its valence electrons are held very, very tightly by the nucleus.
Beryllium has a very small atomic radius, which implies that its electrons are very close to the nucleus. Moreover, the two valence electrons are not effectively screened by the two core electrons, so beryllium does not form cations.
This is why the question was given in the form "If it were to give up its valence electrons", not when it gives up its valence electrons.

