What happens when aqueous solutions of calcium chloride and of sodium carbonate are mixed?
1 Answer
Oct 19, 2015
A double replacement reaction takes place.
Explanation:
Calcium chloride,
The balanced chemical equation for this double replacement reaction looks like this
#"CaCl"_text(2(aq]) + "Na"_2"CO"_text(3(aq]) -> "CaCO"_text(3(s]) darr + 2"NaCl"_text((aq])#
Once you mix the two aqueous solutions, a white insoluble solid, calcium carbonate, will precipitate out of solution.
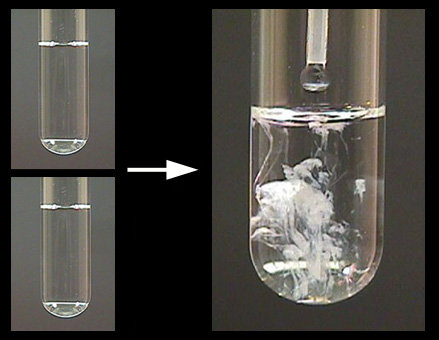
The net ionic equation for this reaction looks like this
#"Ca"_text((aq])^(2+) + "CO"_text(3(aq])^(2-) -> "CaCO"_text(3(s]) darr#

