Question #89d76
1 Answer
Dec 23, 2016
Depending on the number of valence electrons, depends on what group it'll be placed in with elements of the same chemical properties.
Explanation:
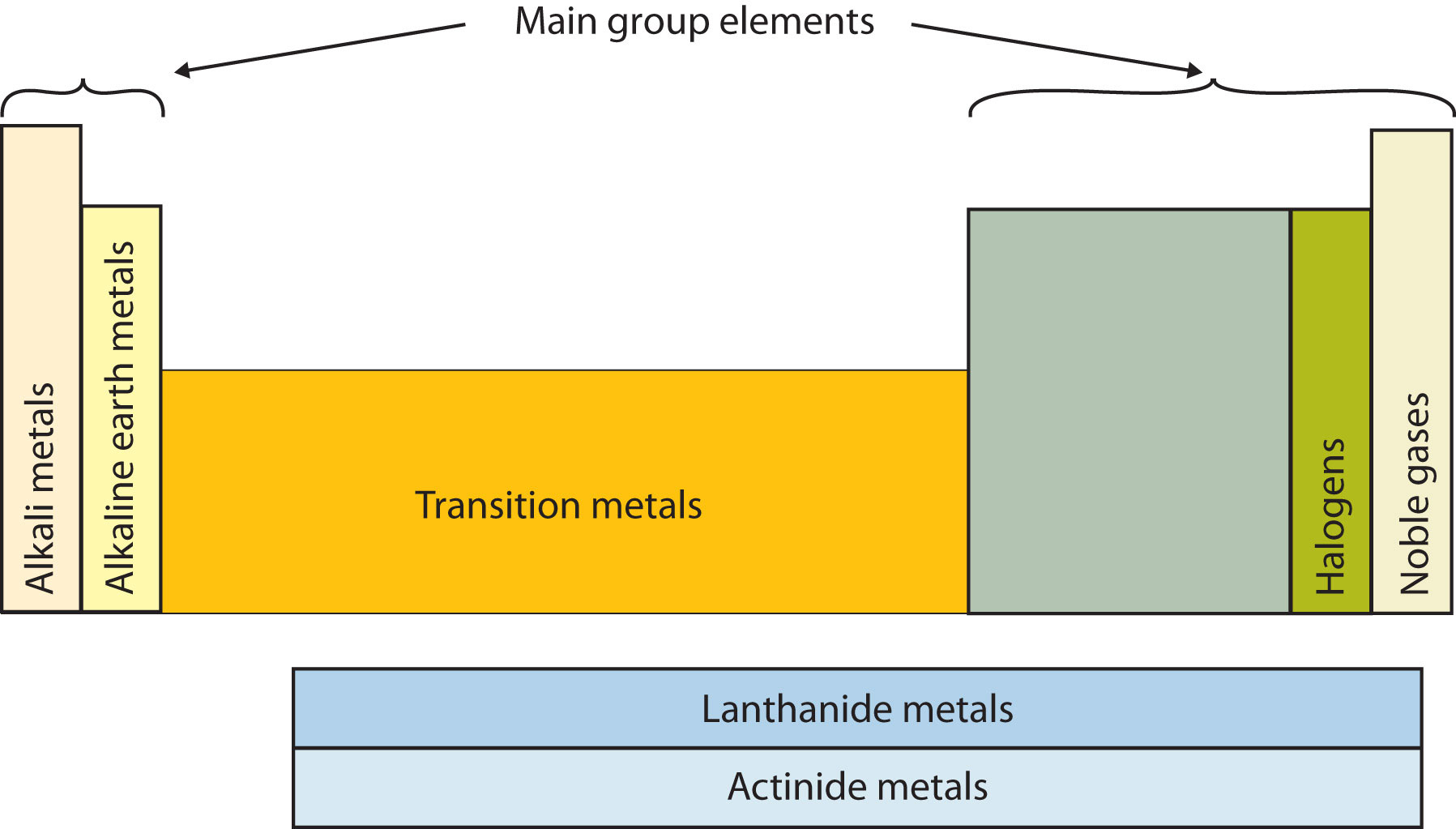
Elements are placed on the periodic table in groups based on how many valence electrons they have.
Group One has only elements with one valance electron, group two has elements with only two valence electrons, and so on to groups 13-18 with 3-8 valence electrons.
Since they are grouped together they share some of the same properties. E.g. Elements with eight valence electrons, the Noble Gases, share the same chemical properties of being nonflammable under normal conditions, and that they are odorless and tasteless.

