How do you go from chloroform to methylacetylene in as few steps as possible?
2 Answers
Nov 1, 2015
pl refer the conversion
Explanation:
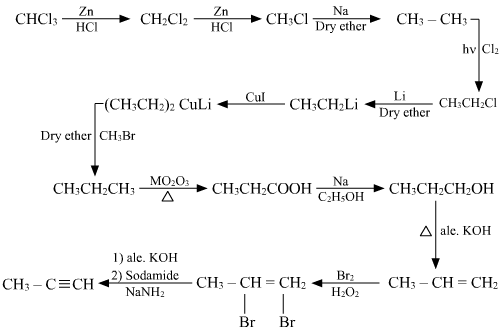
i didnt come up with this answer. i found this over the internet.
here's the link and all credit goes to the person who wrote this. no copying intended =)
Nov 2, 2015
There's a simpler and much cheaper way, if we're going completely theoretical.
- Just put chloroform into solution with 1 equivalent of ethyllithium (the lithium is a fantastic lewis acid) to extend the alkyl chain. You can find ethyllithium in an ethyllithium / benzene solution.
- Two equivalents of tert-butoxide in tert-butanol solvent and raise the temperature to promote two instances of
E2 (bulky nucleophile is terrible at substitution). The alcohol solvent ends up cleaning up any remaining ethyllithium by protonating the ethyl anion
Or, here's a less experimental way of doing it.
- React with 1 equivalent of magnesium in dry ether to make a chlorogrignard reagent, a good nucleophile
- React that with an equivalent of ethyl chloride, a good electrophile. Then the water cleans up any remaining
"MgCl"^(+) and turns it into"Mg"("OH")_2(s) - Two equivalents of tert-butoxide in tert-butanol solvent and raise the temperature to promote two instances of
E2 (bulky nucleophile is terrible at substitution).


