Question #023ed
1 Answer
Lithium chloride.
Explanation:
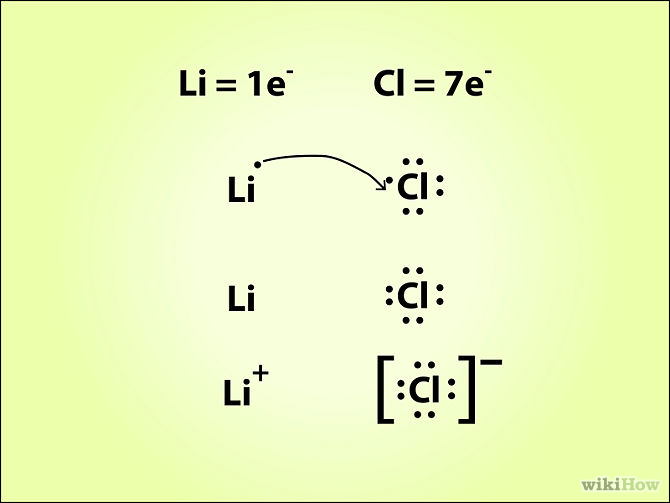
You know that when ionic compounds are formed, one atom is losing electrons to become a positively charge ion and the other is gaining electrons to form a negatively charged ion.
In your case, lithium,
Chlorine, which is located in period 3, group 17 of the periodic table, has seven valence electrons.
When the two atoms bond, lithium will lose its electron and form the lithium cation,
The resulting compound is called lithium chloride.
The name of the ionic compound includes the name of the cation and the name of the anion followed by the suffix -ide.
More on that here:

