Question #6732d
1 Answer
Here's what's going on.
Explanation:
Bromine exists as a molecule rather than as a gas because it is extremely unstable in latter form.
Bromine is located in period 4, group 17 of the periodic table, and has an atomic number equal to
This means that a neutral bromine atom wil have a total of
Now, atoms tend to unstable with outermost shells that have fewer than
In bromine's case, it only lacks
To do that, a bromine atom forms a covalent bond with another bromine atom. Each atom contributes one electron to the bond, and share these bonding electrons equally.
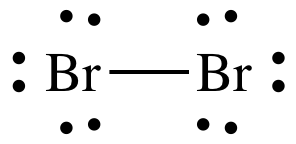
Hence, the bromine molecule,
The interesting thing about bromine is that it is actually a liquid at room temperature.
That happens because its molecules exhibit relatively stronger London dispersion forces caused by the instantaneous and random polarizations of bromine's electron cloud.
Here's how a sample of bromine would look like at room temperature
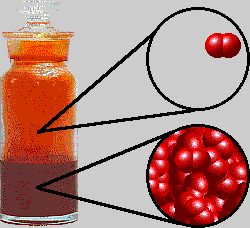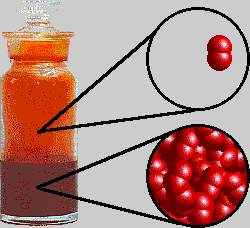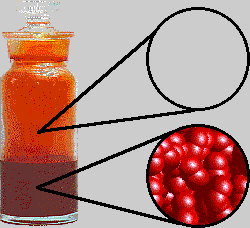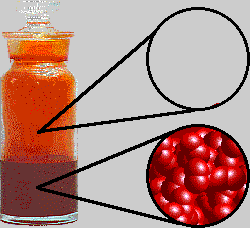
Bromine boils at a little under

