Question #3d3b7
2 Answers
Sulfur Dioxide is made up of only Covalent bonds.
Explanation:
To determine whether a bond is Covalent or Ionic, you must know the difference in electronegativity (a fancy word for describing how good a job a particular element does at attracting new electrons).
An electronegativity difference of anything from 0 (two of the same element bonding together) to 1.69 (a bond that is ALMOST ionic) will result in a covalent bond.
As a side note, the larger the electronegativity difference, the more polar the bond will be.
Since Oxygen has an electronegativity of 3.5 and sulfur has an electronegativity of 2.6, the difference is 0.9.
So the bond is somewhat polar, but still covalent.
Yes, it is!
Explanation:
Covalent compounds are formed when atoms share electrons to complete their octet.
Now, when two atoms that have similar electronegativities bond, the small difference between their respective electronegativities leads to a sharing of the bonding electrons.
As you know, electronegativity gives you an idea about how good an atom is at attracting bonding electrons, which are electrons shared by two atoms when they form a chemical bond.
Now, sulfur and oxygen are both nonmetals, which means thay they have high electronegativity values when compared with metals.
So even if you don't know the exact electronegativities, you can always say that two nonmetals will form a covalent bond.
When a compound contains solely covalent bonds, then you can safely say that you're dealing with a covalent compound.
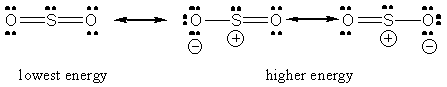
In this case, one way in which these three atoms bond will look like this
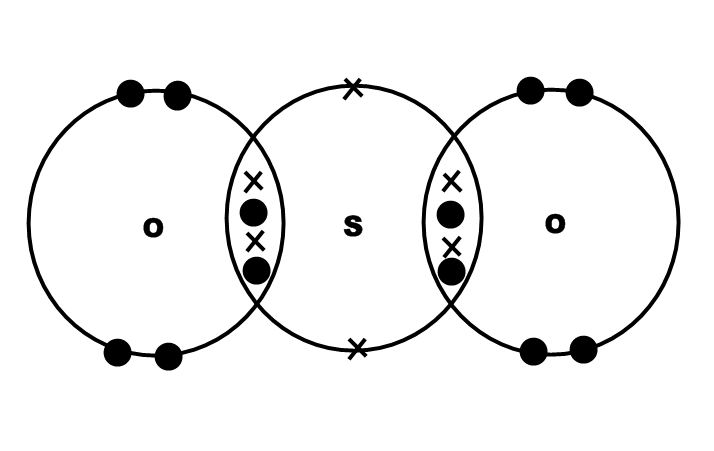
Sulfur shares a total of four valence electrons with the two oxygen atoms. Each oxygen atom shares two valence electrons with sulfur.
The three atoms will share a total of eight valence electrons, the equivalent of two double bonds.
There are other ways in which these three atoms can bond, you can read more on that here
http://socratic.org/questions/what-is-the-lewis-structure-for-so2
but all of them feature shared bonding electrons, which is the characteristic of a covalent compound.