Question #65b15
1 Answer
Hydrogen has a
Explanation:
I don't know who told you this, but hydrogen does not have an oxidation number of
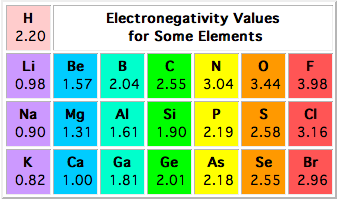
Oxidation states are assigned by taking into account the difference in electronegativity between the two bonded atoms.
The idea is that in a covalent bond, the more electronegative atom is assumed to "take" the bonding electrons. This will leave that atom with more electrons than it had and the less electronegative atom with less electrons than it had before the bond was formed.
Carbon is actually more electronegative than hydrogen, which means that in a
Methane has one carbon atom bonded to four hydrogen atoms via single bonds.
If carbon is to take both bonding electrons from each of those four bonds, it will end up with four extra electrons, which are the electrons the hydrogen atoms were providing to the bond.
This will give carbon a