Question #c6d7b
1 Answer
Explanation:
The trick here is to recognize that copper(II) ions actually exist as a complex ions in aqueous solution.
The simplest complex ion that copper(II) cations form in aqueous solution is the hexaaquacopper(II) ion,
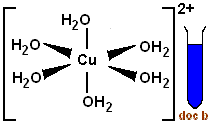
Now, when concentrated hydrochloric acid,
Now, you need to keep track of the charge on the complex ion. Because the aqua ligands are neutral molecules, the charge of the hexaaquacopper(II) ion will be the same as the charge of the copper(II) cation.
These six neutral ligands are replaced by four anions, each carrying a
The reaction will produce the tetrachlorocopper(II) ion,
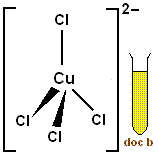
The balanced net ionic equation for this reaction will look like this
#["Cu"("H"_2"O")_6]_text((aq])^(2+) + 4"Cl"_text((aq])^(-) -> ["CuCl"_4]_text((aq])^(2-) + 6"H"_2"O"_text((l])#

