On average, how many #pi# bonds does #"O"_2^(+)# have?
2 Answers
As mo theory there should be 1.5 pi bond
Explanation:
MO configuration of
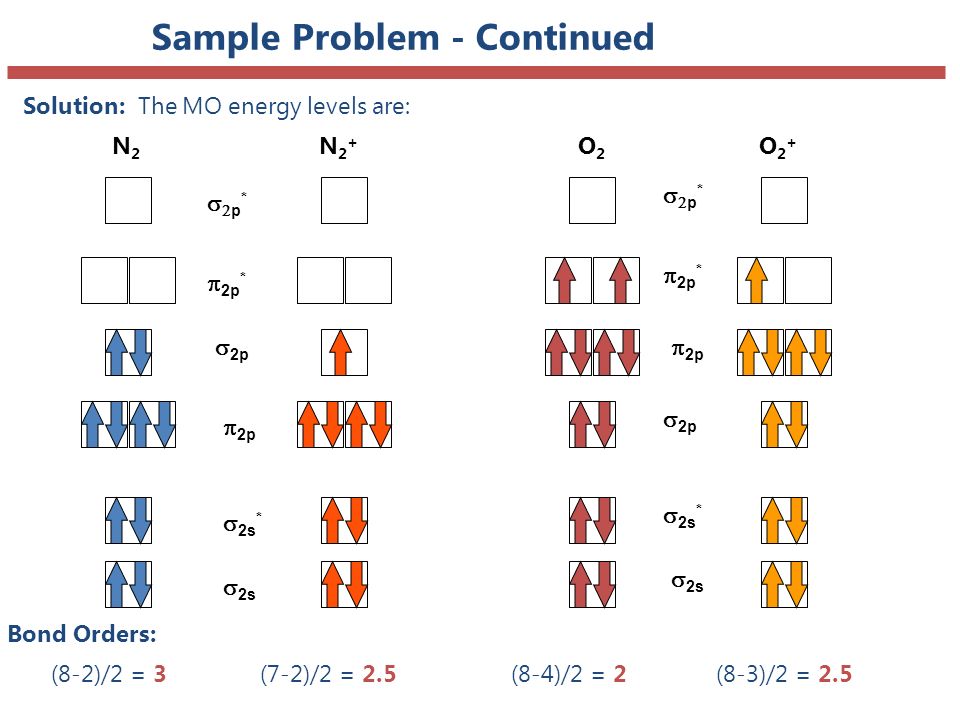
You would have a bond order of
BOND ORDER OF HOMONUCLEAR DIATOMICS
Bond order is a measure of bond strength, and suggests stability. It is half the number of bonding minus the number of antibonding molecular orbitals.
#"Bond Order" = ("Bonding e"^(-) - "Antibonding e"^(-))/2#
You can have optimal overlap, less than optimal overlap, or no overlap. Poorer bonding overlaps correspond with lower values of bond order. Or, less antibonding overlap corresponds with higher values of bond order (which is what applies here).
The structures of
DIATOMIC OXYGEN IS PARAMAGNETIC
While Valence Bond Theory suggests oxygen is diamagnetic, Molecular Orbital Theory correctly demonstrates that oxygen,
That means it has unpaired electrons. Specifically, two unpaired electrons, one in each
The MO diagram for neutral
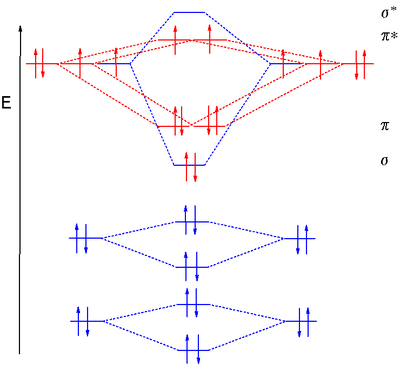
When you take away one electron, you take it away from the highest-occupied molecular orbital. Since either the
DETERMINING BOND ORDER
Naturally,
Two bonding electrons each come from the
#(10 - color(red)(6))/2 = color(blue)(2)#
When taking away one
#(10 - color(red)(5))/2 = color(blue)(2.5)#
Since


