Question #17fa2
1 Answer
Dec 2, 2015
Explanation:
As you know,
In order to get the number of valence electrons for a molecule, you need to take into account the number of valence electrons each atom that's a part of that molecule brings to the table.
So, the hydrogen molecule is comprised of two hydrogen atoms. A neutral hydrogen atom has one proton and one electron.
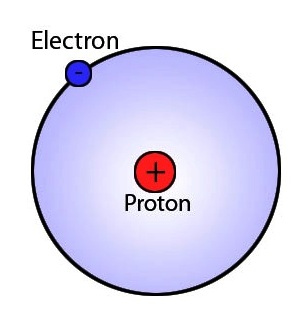
This solitary electron will serve as hydrogen's valence electron. If each hydrogen atom brings one valence electron to the molecule, the you can say that the hydrogen molecule,

