Question #c698b
1 Answer
Explanation:
Your strategy here will be to use the definition of the unified atomic mass unit,
Once you know the mass of a cell, use nickel's density to determine the cell's volume.
So, the unified atomic mass unit is defined as
#"1 u" = 1.660539 * 10^(-24)"g"#
The mass of a single nickel atom expressed in grams will thus be
#58.7 color(red)(cancel(color(black)("u"))) * (1.660539 * 10^(-24)"g")/(1color(red)(cancel(color(black)("u")))) = 9.747 * 10^(-23)"g"#
Now, a face-centered cubic cell structure is characterized by a total of
- one lattice point in every corner of the cell
#-># a total of eight corner lattice points- one lattice point in every face of the cell
#-># a total of six face lattice points
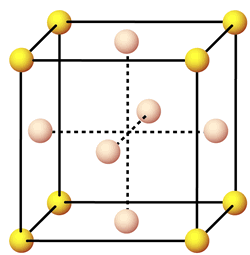
Each corner lattice point will hold
#"no. of atoms" = overbrace(1/8 * 8)^(color(blue)("corners")) + overbrace(1/2 * 6)^(color(red)("faces")) = "4 atoms"#
So, if each cell contains a total of
#4 color(red)(cancel(color(black)("atoms"))) * (9.747 * 10^(-23)"g")/(1color(red)(cancel(color(black)("atom")))) = 3.899 * 10^(-22)"g"#
Use the given density to find the volume of a unit cell
#3.899 * 19^(-22) color(red)(cancel(color(black)("g"))) * "1 cm"^3/(8.9 color(red)(cancel(color(black)("g")))) = 4.381 * 10^(-23)"cm"^3#
As you know, the volume of a cube is given by the formula
#color(blue)(V = l xx l xx l = l^3)" "# , where
This means that you have
#l = root(3)(V)#
In your case, this is equivalent to
#l = root(3)(4.381 * 10^(-23)"cm"^3) = 3.525 * 10^(-7)"cm"#
The length of the unit cell is usually given in nanometers, so convert the result to get
#3.525 * 10^(-8) color(red)(cancel(color(black)("cm"))) * (10^7"nm")/(1color(red)(cancel(color(black)("cm")))) = "0.3525 nm"#
Rounded to two sig figs, the number of sig figs you have for nickel's density, the answer will be
#l = color(green)("0.35 nm")#

