Can we figure out how many sigma and pi bonds are in a molecule without drawing it out?
1 Answer
It's extremely difficult without knowing the bonding preferences of each atom or making the structure. It can be hard to grasp at first, but it's something that you will find useful to know in future chemistry classes, so it's best to learn it as soon as possible.
For simpler molecules, you might be able to get by without drawing the structure, but sooner or later you will be considering the connection of atoms in space and eventually the overall structure.
EXAMPLE: DIATOMIC NITROGEN
Let's say that for some reason we don't want to draw out the structure until we figure out how many
For example,
Since all three
Thus, we form a triple bond, which consists of the one
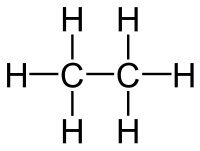
Now that we have the structure, we simply say that:
- Each single bond contains one
#sigma# bond - Each double bond contains one
#sigma# bond and one#pi# bond - Each triple bond contains one
#sigma# bond and two#pi# bonds
So, you can point to each single bond and say that this molecule has
EXAMPLE: SOMETHING CRAZY
And now, an example of something where there is no way anyone can ever figure out the number of
This molecule has the structural formula
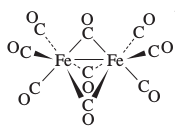
That's insane! Now, if we expanded this structure to show all the bonds, we would get:
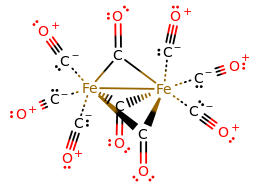
And it would be preposterous to try to figure out the number of

