Explain how convection, conduction, phase transitions, average kinetic energy, and equilibrium are related?
1 Answer
Well, I'll see what I can do, but what you're asking for is a lot for one question.
As a heads-up, convection mainly occurs within one-phase fluids of low density, e.g. gases (or sometimes liquids). Convection is mainly internal fluid flow so that hotter regions transfer heat into previously colder regions.
This doesn't really have to do with phase changes, and is only what happens while fluids reach thermal equilibrium (i.e. when their temperature distribution works to become uniform).
- Phase transitions always occur at constant temperature WHILE the phase change is going on. This temperature is known as, e.g. melting point, boiling point, etc.
It is at this temperature that the two phases involved (e.g. solid + liquid for melting point, liquid + gas for boiling point, etc) are in phase equilibrium, i.e. the rate of the forward process equals the rate of the reverse process.
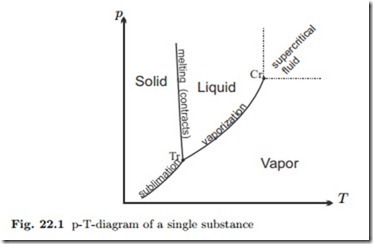
(Here, the phase equilibrium is during the moment when we are crossing the lines that define the boundaries of each phase on the phase diagram. Typically we move horizontally, meaning we are heating/cooling at constant pressure.)
- If we are heating, all the heat energy that goes in (via conduction, mostly, i.e. heat flow through touch) makes the molecules move faster, breaking (to some extent) electrostatic forces of attraction that keep them together, and the molecules can then move more freely.
All that heat is "invested" in making the phase change occur, and only afterwards will it change the temperature of the substance.
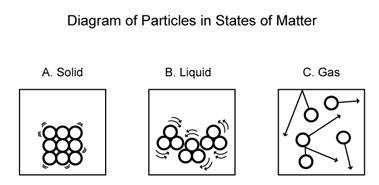
You can see in the above diagram that solids tend to be rigid with little to no motion. Liquids have a bit more freedom, a little more loose, but not much free range...
Gases have the most degrees of freedom of these three phases, and therefore have the highest kinetic energy among these phases of matter. Barely any intermolecular forces hold them together.

