How do you construct a Born-Haber cycle to calculate the lattice energy of sodium chloride?
1 Answer
By definition, the gaseous cation and anion forming the corresponding ionic compound release energy termed the lattice energy, the energy contained within the lattice structure.
For an alternative explanation, see here.
The Born-Haber cycle takes advantage of the state function property of the change in enthalpy to indirectly determine the lattice energy of ionic compounds through processes that utilize known thermodynamic quantities like ionization energy and electron affinity.
Let's take
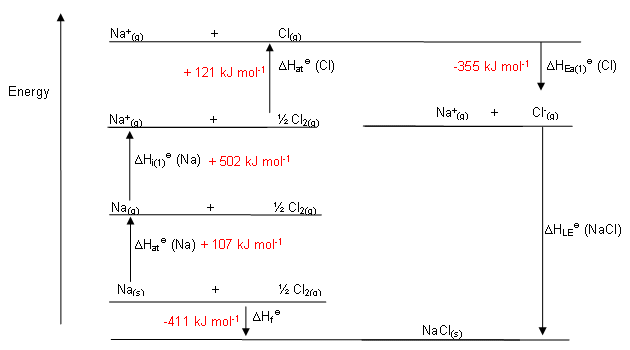
"Na"(s) + 1/2"Cl"_2(g) -> "NaCl"(s)
Our goal is to transform the reactants into their ionic gases, as that is the reaction that describes the process for which "lattice energy" is defined.
"Na"(s) -> "Na"(g) lArr sublimation of sodium solid."Na"(g) -> "Na"^(+)(g) + e^(-) lArr Ionization of the gas to remove an electron is by definition the ionization energy.1/2"Cl"_2(g) -> "Cl"(g) lArr chlorine is now made atomic (defines bond energy)."Cl"(g) + e^(-) -> "Cl"^(-)(g) lArr chlorine was a gas, and now needs to gain an electron, the definition of electron affinity."Na"^(+)(g) + "Cl"^(-)(g) -> "NaCl"(s) lArr the formation of the lattice!
Put this all together, with some data, and we get, for
"NaCl"(s) -> "Na"(s) + 1/2"Cl"_2(g) ,-DeltaH_(f,"NaCl"(s)) = +"411 kJ"
"Na"(s) -> "Na"(g) ,DeltaH_("sub","Na") = "107 kJ"
"Na"(g) -> "Na"^(+)(g) + e^(-) ,"IE"_(1,"Na"(g)) = "502 kJ"
1/2"Cl"_2(g) -> "Cl"(g) ,1/2DeltaH_("bond","Cl"_2(g)) = 1/2xx"242 kJ"
"Cl"(g) + e^(-) -> "Cl"^(-)(g) ,"EA"_(1,"Cl"(g)) = -"355 kJ"
"Na"^(+)(g) + "Cl"^(-)(g) -> "NaCl"(s) ,DeltaH_"lattice" = ???
"-----------------------------------------------------------------------------"
"These cancel out completely upon adding, proving"
"we have a complete cycle."
And now if we wish, the lattice energy can be calculated.
 http://www.4college.co.uk/
http://www.4college.co.uk/
Take the
0 = DeltaH_"cycle" = DeltaH_(f,"NaCl"(s))^@ + DeltaH_("sub","Na") + "IE"_(1,"Na"(g)) + 1/2DeltaH_("bond","Cl"_2(g)) - "EA"_(1,"Cl"(g)) - DeltaH_"lattice"
Solving for
color(blue)(DeltaH_"lattice" -= -|DeltaH_"lattice"|)
= color(blue)(-[DeltaH_(f,"NaCl"(s))^@ + DeltaH_("sub","Na") + "IE"_(1,"Na"(g)) + 1/2DeltaH_("bond","Cl"_2(g)) - "EA"_(1,"Cl"(g))])
where all the numbers you plug in are positive. For example, we'd get:
color(blue)(DeltaH_("lattice","NaCl"(s)))
= -[411 + 107 + 502 + 1/2(242) - 355] "kJ"
= color(blue)(-"786 kJ")

