Question #2bc5b
1 Answer
Rutherford's Model predicted all atoms were unstable. The spectra produced by atoms was continuous.
Bohr's model attempted to fix these issue.
Explanation:
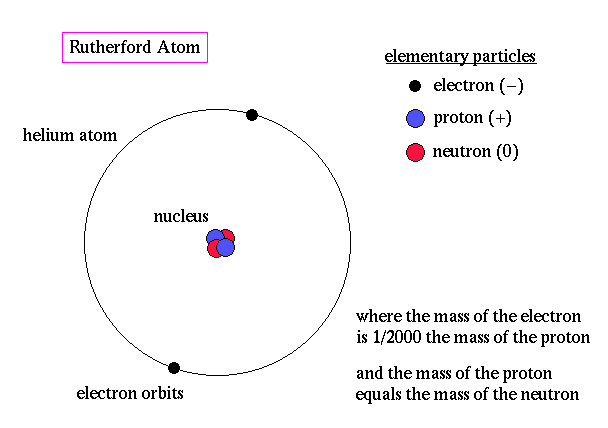
Rutherford model of atom was based on experiment.
He proposed that all the atom's positive charge was concentrated in a very tiny volume at the centre of the atom. And that magnitude of this charge was proportional to the mass number of the atom.
He also proposed that electron cloud revolves around this central part of the atom much like planets rotate around the sun in our solar system, the size of the atom being about
Now we know this to be as shown in the picture.
 abyss.uoregon.edu
abyss.uoregon.edu
In this model there was a technical difficulty. As per Classical mechanics; the negatively charged electrons, as these circled around the positively charged nucleus, would radiate electromagnetic energy. In due course electrons will loose energy and spiral inwards to crash in to the nucleus. Therefore, (1) All atoms were predicted to be unstable (2) Electrons will produce continuous smear of electromagnetic spectrum.
Rutherford-Bohr model, called Bohr Model in short, attempted to address these two shortcomings.
It was known experimentally that atoms emit light at certain discrete frequencies. Bohr thus proposed that
The electrons can only orbit the nucleus stably, in fixed orbits at a fixed distances from the nucleus. These orbits are associated with definite energies and are also called energy shells or energy levels.
While in these orbits, the electron's acceleration does not produce any electromagnetic radiation and energy loss.
As we see it is introduction of quantum mechanics.
Bohr also proposed that any electron can gain or loose energy by jumping from one to another allowed energy levels by absorbing or radiating electromagnetic energy. The frequency
where
Bohr's Model of Atom
 wikispaces.com
wikispaces.com
