Question #16f9d
1 Answer
Here's what I got.
Explanation:
All you really need to know here is that oxygen has an oxidation number of
Likewise, it's good to know that hydrogen will usually have a
So, for hypochlorous acid,
#stackrel(color(blue)(+1))("H")stackrel(color(blue)(-2)) ("O") stackrel(color(blue)(?))"Cl"#
For a neutral molecule, the oxidation numbers of all the atoms that make up the molecule must add up to give zero.
This means that you have
#overbrace(1 xx (+1))^(color(red)("one atom of H")) + overbrace(1 xx (-2))^(color(orange)("one atom of H")) + overbrace(1 xx 1 xx color(blue)(?))^(color(green)("one atom of Cl")) = 0#
You will have
#color(blue)(?) = 0 + 2 - 1 = +1#
Therefore, the oxidation numbers for the three elements that make up hypochlorous acid are
#stackrel(color(blue)(+1))("H")stackrel(color(blue)(-2)) ("O") stackrel(color(blue)(+1))"Cl"#
Now, the exact same approach can be used to find the oxidation numbers for sodium hypochlorite,
The only difference is that you know that sodium, which is a group 1 metal, will always have an oxidation number of
Once again, the oxidation numbers will come out to be
#stackrel(color(blue)(+1))("Na")stackrel(color(blue)(-2)) ("O") stackrel(color(blue)(+1))"Cl"#
Alternatively, you can assign oxidation numbers by looking at the Lewis structure of the molecule.
For example, the Lewis structure for the hypochlorous acid molecule looks like this
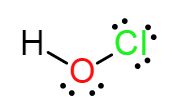
Now, oxidation numbers are assigned by distributing all of the bonding electrons to the more electronegative atom.
So, oxygen is more electronegative than hydrogen, so it will take both bonding electrons it shares with hydrogen. This will give it a
Oxygen is also more electronegative than chlorine, which means that it will both bonding electrons from the bond it has with chlorine.
This will get oxygen's oxidation number to
Since both hydrogen and chlorine "lost" an electron, their oxidation numbers will be

