Question #22019
1 Answer
Liquid
Explanation:
The question asks which liquid will be distilled first, which is actually equivalent to asking which liquid has the lowest boiling point.
This, in turn, goes back to the intermolecular forces of attraction, more specifically to the strength of the intermolecular forces of attraction, each molecule exhibits.
So basically, the question wants you to say which molecule exhibits the weakest intermolecular forces of attraction.
As you know, the boiling point of a liquid is directly related to how strong its molecules are attracted to each other.
All the four molecules listed are nonpolar molecules, which means that they only exhibit London dispersion forces, the weakest type of intermolecular forces.
Now, the relative strength of the London dispersion forces depends on the size of a molecule's electron cloud. London dispersion forces (LDF) are characterized by instantaneous and random changes in the electron cloud.
These changes lead to the formation of temporary partial charges known as instantaneous dipoles. These dipoles induce similar dipoles in neighboring molecules, thus creating an electrostatic attraction between molecules.
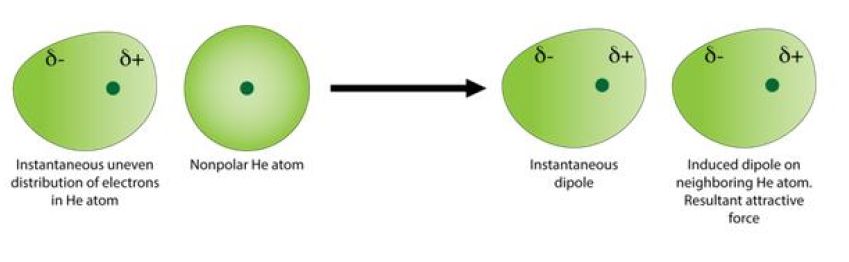
The strength of these instantaneous dipoles will depend on the size of the electron cloud. The bigger the electron cloud, the more powerful these instantaneous dipoles will be.
Since the size of the electron cloud is directly connected to the size of the molecule, you can say that the smallest molecule on the list will also have the smallest electron cloud, i.e. the fewest electrons, and thus the weakest LDF's.
In this case, the hydrogen molecule is the smallest of the bunch. Its electron cloud only contains

