Question #7f583
1 Answer
Here's what I got.
Explanation:
The key to this problem is the balanced chemical equation for this neutralization reaction.
Since you're in aqueous solution, you can simplify the equation by removing the sodium cations,
#"HX"_text((aq]) + "NaOH"_text((aq]) -> "X"_text((aq])^(-) + "H"_2"O"_text((aq])#
Notice that you have
This tells you that for every one mole of strong base added to the weak acid solution, one mole of weak acid is consumed and one mole of conjugate base is produced.
So, your initial solution contains
#1/3 * n -># one third of the number of moles of weak acid
In order to consume
#n_"sol B" = n - n/3 = color(green)((2n)/3)#
Remember that consuming
#"For HX: " (2n)/3" moles"#
#"For X"^(-): color(white)(a)n/3" moles"#
At this point, you should be able to tell that the concentration of the weak acid is twice as high as the concentration of the conjugate base in solution B. Nevertheless, here's how you can prove that.
Let's assume that the volume of solution B is equal to
#color(blue)(c = n/V)#
#["HX"] = (2n)/3"moles" * 1/"V L" = ((2n)/3 * 1/V) " M"#
#["X"^(-)] = n/3"moles" * 1/"V L" = (n/3 * 1/V)" M"#
This means that you have
#(["HX"])/(["X"^(-)]) = ((2n)/3 * color(red)(cancel(color(black)(1/V " M"))))/(n/3 * color(red)(cancel(color(black)(1/V " M")))) = (2 color(red)(cancel(color(black)(n))))/3 * 3/color(red)(cancel(color(black)(n))) = color(green)(2)#
Now, in order to find the acid dissociation constant,
#"HX"_text((aq]) + "H"_2"O"_text((aq]) rightleftharpoons "H"_3"O"_text((aq])^(+) + "X"_text((aq])^(-)#
By definition,
#K_a = (["H"_3"O"^(+)] * ["X"^(-)])/(["HX"]) = (["X"^(-)])/(["HX"]) * ["H"_3"O"^(+)]#
Since you know that
#["H"_3"O"^(+)] = 4.2 * 10^(-4)"M"#
and that
#(["HX"])/(["X"^(-)]) = 2 <=> (["X"^(-)])/(["HX"]) = 1/2#
you can say that
#K_a = 1/2 * 4.2 * 10^(-4) = color(green)(2.1 * 10^(-4))#
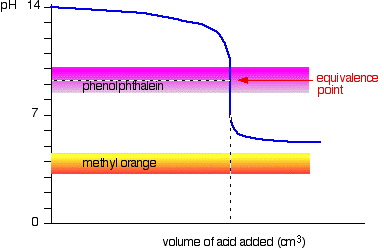
Finally, methyl orange is not a suitable indicator for this titration because at the equivalence point,m the pH of the resulting solution is higher than
This happens because when all of the weak acid is neutralized, the remaining solution will still contain its conjugate base
Methyl orange changes color in the

which means that it will not be useful for your weak acid - strong base titration. Instead, you should pick an indicator that changes color above