Question #989c7
1 Answer
Here's what I got.
Explanation:
Ethanedioic acid, also known as oxalic acid,
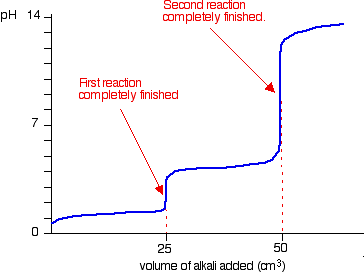
Its titration curve shows two equivalence points.
The first equivalence point corresponds to the complete deprotonation of the first acidic proton to leave behind
The second equivalence point corresponds to the complete deprotonation of the second acidic proton to leave behind
So, the reaction that is completed at the first equivalence point will be
#"H"_2"C"_2"O"_text(4(aq]) + "OH"_text((aq])^(-) -> "HC"_2"O"_text(4(aq])^(-) + "H"_2"O"_text((l])#
The reaction that is completed at the second equivalence point will be
#"HC"_2"O"_text(4(aq])^(-) + "OH"_text((aq])^(-) -> "C"_2"O"_text(4(aq])^(2-) + "H"_2"O"_text((aq])#
To get the balanced chemical equation for the overall reaction, add these two reactions
#"H"_2"C"_2"O"_text(4(aq]) + "OH"_text((aq])^(-) -> color(red)(cancel(color(black)("HC"_2"O"_text(4(aq])^(-)))) + "H"_2"O"_text((l])#
#color(red)(cancel(color(black)("HC"_2"O"_text(4(aq])^(-)))) + "OH"_text((aq])^(-) -> "C"_2"O"_text(4(aq])^(2-) + "H"_2"O"_text((l])#
#color(white)(aaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaa)/color(white)(aaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaa)#
#"H"_2"C"_2"O"_text(4(aq]) + color(purple)(2)"OH"_text((aq])^(-) -> "C"_2"O"_text(4(aq])^(2-) + 2"H"_2"O"_text((l])#
Use the molarity and volume of the sodium hydroxide solution to find how many moles of hydroxide anions were needed to get to the second equivalence point
#color(blue)(c = n/V implies n = c * V)#
#n_(OH^(-)) = 0.0450"moles"/color(red)(cancel(color(black)("dm"^3))) * 41.6 * 10^(-3)color(red)(cancel(color(black)("dm"^3)))#
#n_(OH^(-)) = "0.001872 moles OH"^(-)#
Since you have a
#0.001872 color(red)(cancel(color(black)("moles OH"^(-)))) * ("1 mole H"_2"C"_2"O"_4)/(color(purple)(2)color(red)(cancel(color(black)("moles OH"^(-))))) = "0.000936 moles H"_2"C"_2"O"_4#
Finally, use the known volume of the acid to find its molarity
#c = "0.000936 moles"/(25.0 * 10^(-3)"dm"^3) = color(green)("0.0374 mol dm"^(-3))#
The answer is rounded to three sig figs.