When do you use parentheses when naming transition metal complexes?
1 Answer
There's no hard and fast rule; generally, for more complex ligands, like polydentates (chelating ligands), you would.
When there is more than one complex ligand (not tooth, but ligand), you put bis, tris, tetrakis, pentakis, etc. because it looks better than something like di(diethylenediamine) or di(dialkyldithiocarbamato). Use your best judgment.
Some examples and their formula abbreviations are:
- ethylenediamine (en) ~ bidentate (contributes
#0# )
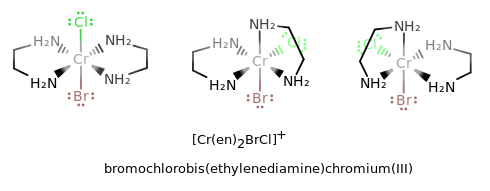
where the first ligand is a trans isomer, the second is a
#Lambda# isomer, and the third is a#Delta# isomer. (Rotate the second isomer clockwise#90^@# and the third isomer counterclockwise#90^@# to get the standard orientation.)
In this case, since there are two ethylenediamines, but the ligand already has "di" in it (and is complex), we put "bis" because it sounds good.
- 1,10-phenanthroline (o-phen / phen) ~ bidentate (contributes
#0# )
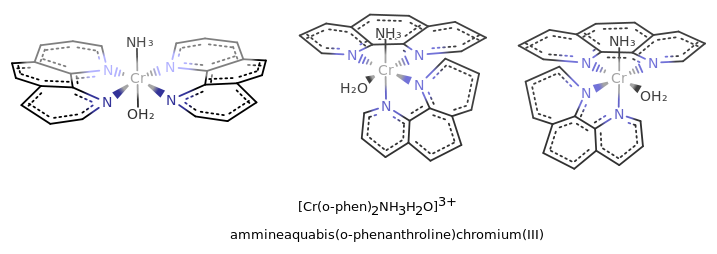
where the first structure is a trans isomer, the second is a
#Delta# isomer, and the third is a#Lambda# isomer. (Flip the second two isomers upside-down to get the standard orientation.)
In this case, the name is just complex, and the ligand is bidentate, so we write "bis(o-phenanthroline)" in the name but
- dialkyldithiocarbamato (dtc) ~ bidentate (contributes
#-1# )
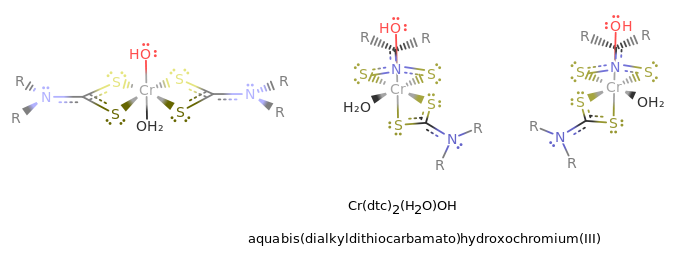
where the first structure is a trans isomer, the second is a
#Delta# isomer, and the third is a#Lambda# isomer. (Flip the second two isomers upside-down to get the standard orientation.)
The
- oxalato (ox) ~ bidentate (binds via oxygens in an acetate resonance structure; contributes
#-2# )
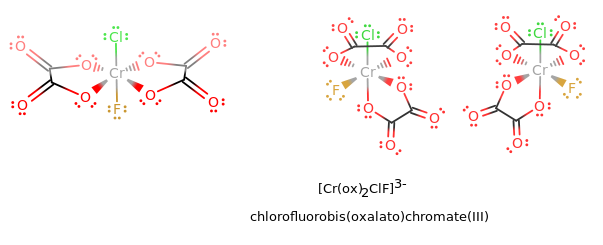
where the first structure is a trans isomer, the second is a
#Delta# isomer, and the third is a#Lambda# isomer. (Flip the second two isomers upside-down to get the standard orientation.)
Each oxalato ligand contributes

