Question #c6de3
1 Answer
Explanation:
This problem wants to test your knowledge of which ionic compounds are soluble and which are insoluble in aqueous solution.
In order to be able to answer this question, you must be familiar with the solubility rules for ionic compounds in aqueous solution
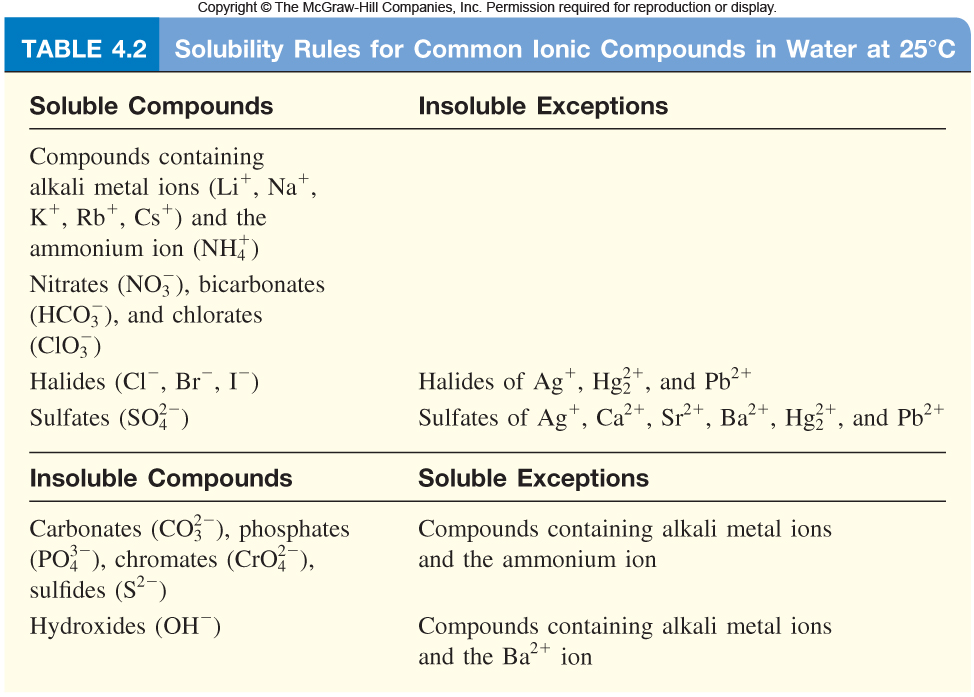 http://highered.mheducation.com/olcweb/cgi/pluginpop.cgi?it=jpg::::::/sites/dl/free/0023654666/650262/Solubility_Rules_4_02.jpg::Solubility%20rules
http://highered.mheducation.com/olcweb/cgi/pluginpop.cgi?it=jpg::::::/sites/dl/free/0023654666/650262/Solubility_Rules_4_02.jpg::Solubility%20rules
So, you know that your solution is treated with potassium chloride,
All of these ionic compounds are soluble in aqueous solution, which means that they exist as cations and anions when dissolved in water
"KCl"_text((aq]) -> "K"_text((aq])^(+) + "Cl"_text((aq])^(-)
"Na"_2"SO"_text(4(aq]) -> 2"Na"_text((aq])^(+) + "SO"_text(4(aq])^(2-)
"NaOH"_text((aq]) -> "Na"_text((aq])^(+) + "OH"_text((aq])^(-)
You're looking for cations, which are positively charged ions, that will combine with the sulfate anions,
Moreover, you need these cations to not form an insoluble solid with the chloride anions,
As you can see in the list, the only cation that is of interest here is the barium cation,
Barium cations will not form an insoluble solid with the chloride anions, since barium chloride,
Likewise, barium hydroxide,
However, barium cation will react with the sulfate anions to form barium sulfate,
 http://fphoto.photoshelter.com/image/I0000FlOVtJqG.ik
http://fphoto.photoshelter.com/image/I0000FlOVtJqG.ik
Therefore, your unknown soluble ionic compound most likely contains barium cations,

