Question #9b08b
1 Answer
There are four phases of matter:
- Solids
- Liquids
- Gases
- Plasmas
Explanation:
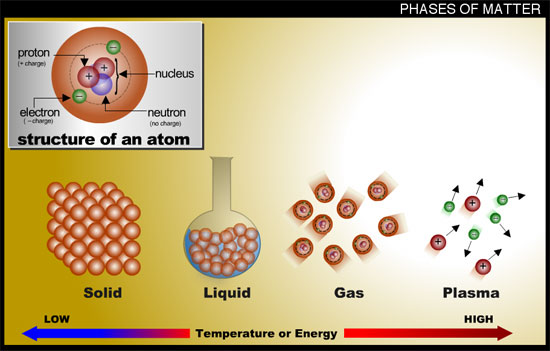
1. Solids. Individual atoms / molecules in the substance are strongly bound close together. Atoms / molecules vibrate around an equilibrium position. Solids to not adjust their shape to fill their container.
2. Liquids. Individual atoms / molecules are weakly bound together, the mean separation may be slightly larger than that of the solid phase. The atoms / molecules are able to slip past each other ( translational motion ). Therefore liquids will flow to fill their container in the horizontal direction.
3. Gases. Individual atoms / molecules are not bound together – their motion is fully translational. Gases will therefore fill their continuer in all three dimensions, i.e. they will fill the whole volume of their container.
4. Plasmas. Similar to gases but the atoms / molecules are ionised. Some electrons are disassociated from their parent atoms giving rise to a mixture of cations* and disassociated electrons. Photons are also produced in plasmas. See footnote below for some examples of plasmas.
*Cations are positively charged ions (either ionised atoms or ionised molecules.
**Plasmas are the least commonly known of the phases of matter so here are some examples of where they occur:
• Lightning strikes, the charged particles allow the high voltage of the clouds to conduct to Earth. Photons of light are also emitted, which is why we can see lightning strikes.
• The Sun (and other stars) consist of plasma.
• Flames are considered to be a plasma.


