Question #eb9d1
1 Answer
First understand what an Isotope is:
Isotopes can be defined as the atoms of a same element or the species of an atom with different number of Neutrons but same protons (Atomic number)
Example:
The 3 Isotopes of Hydrogen
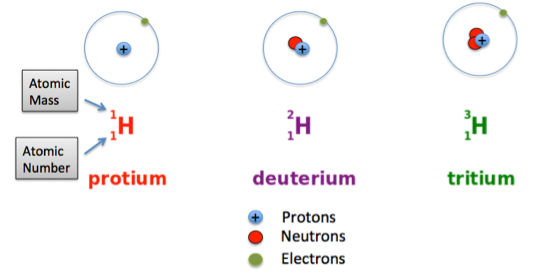
Thank you Tyler Dewitt
So,
Radioactive Isotopes are Isotopes that emit radiation in the form of particles or energy
They are Radioactive,because their Nuclei is unstable
Example (Radioactive Isotopes):
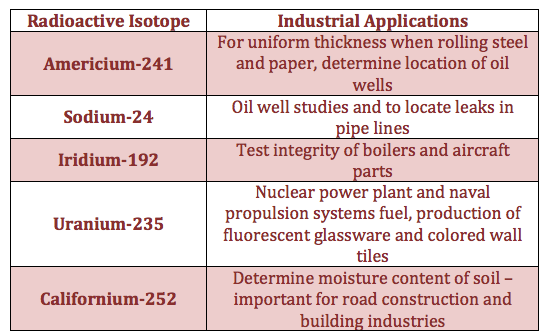
The radiations emitted:
3 types:
I am answering the question asked in the comments:
Why the atoms are unstable?
(some) Atoms are unstable because, they do not have the energy to hold together their nuclei.
And release the energy in the form of Alpha,Beta and Gamma particles
The particles are made of Neutrons and Protons

