Question #72819
1 Answer
Explanation:
Gallium-72 will undergo radioactive decay via beta minus decay,
Beta minus decay occurs when the nucleus of a radioactive nuclide emits an electron,
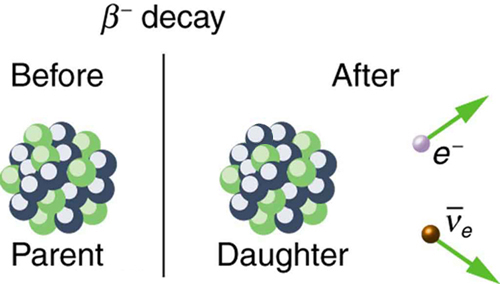
This happens because a neutron located inside the nucleus is converted to a proton.
#color(purple)(|bar(ul(color(white)(a/a)color(black)(""_ 0^1"n" -> ""_ 1^1"p" + ""_ "-1"^0"e" + bar(nu)_"e")color(white)(a/a)|)))#
As a result, the atomic number of the daughter nuclide will increase by
On the other hand, its mass number, which represents the number of protons and neutrons located inside the nucleus, will remain unchanged.
So, you know that gallium-72 has a mass number equal to
You'll find gallium located in period 3, group 13. Its atomic number is equal to
Germanium,
The beta decay equation will look like this
#color(green)(|bar(ul(color(white)(a/a)color(black)(""_ 31^72"Ga" -> ""_ 32^72"Ge" + ""_ "-1"^0"e" + bar(nu)_"e")color(white)(a/a)|)))#

