Beta Decay
Key Questions
-
Beta decay represents the transformation of a neutron from the nucleus of a radioactive element into a proton, an electron, also called a beta particel, and an antineutrino.
The electron and the antineutrino are emitted from the nuclues, which now has one extra proton; this essentially changes the element, since the atomic number has now increased by 1.
There are two types of beta decay, beta-minus, which I described in the previous paragraph, and beta-plus, in which a proton decays into a neutron, a positron, and a neutrino.
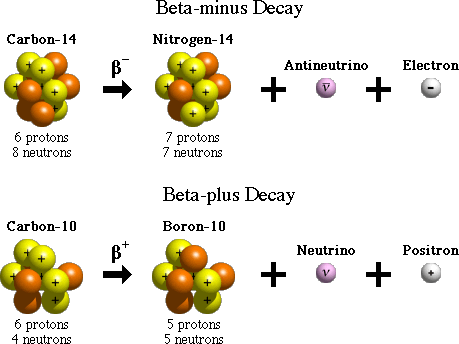
Both reactions occur when an atom has either too many protons, or too many neutron in its nucleus. One type of beta decay or the other will move the atom closer to the region of stability in the Chart of Nuclides, essentially bringing the atom closer to the optimal ratio between protons and neutrons.
The Chart of Nuclides represents a two-dimensional graph which shows an atom's number of protons on one axis ,and the number of neutrons on the other. This system provides a great insight into the characteristics of isotopes.
Here's what this chart looks like:
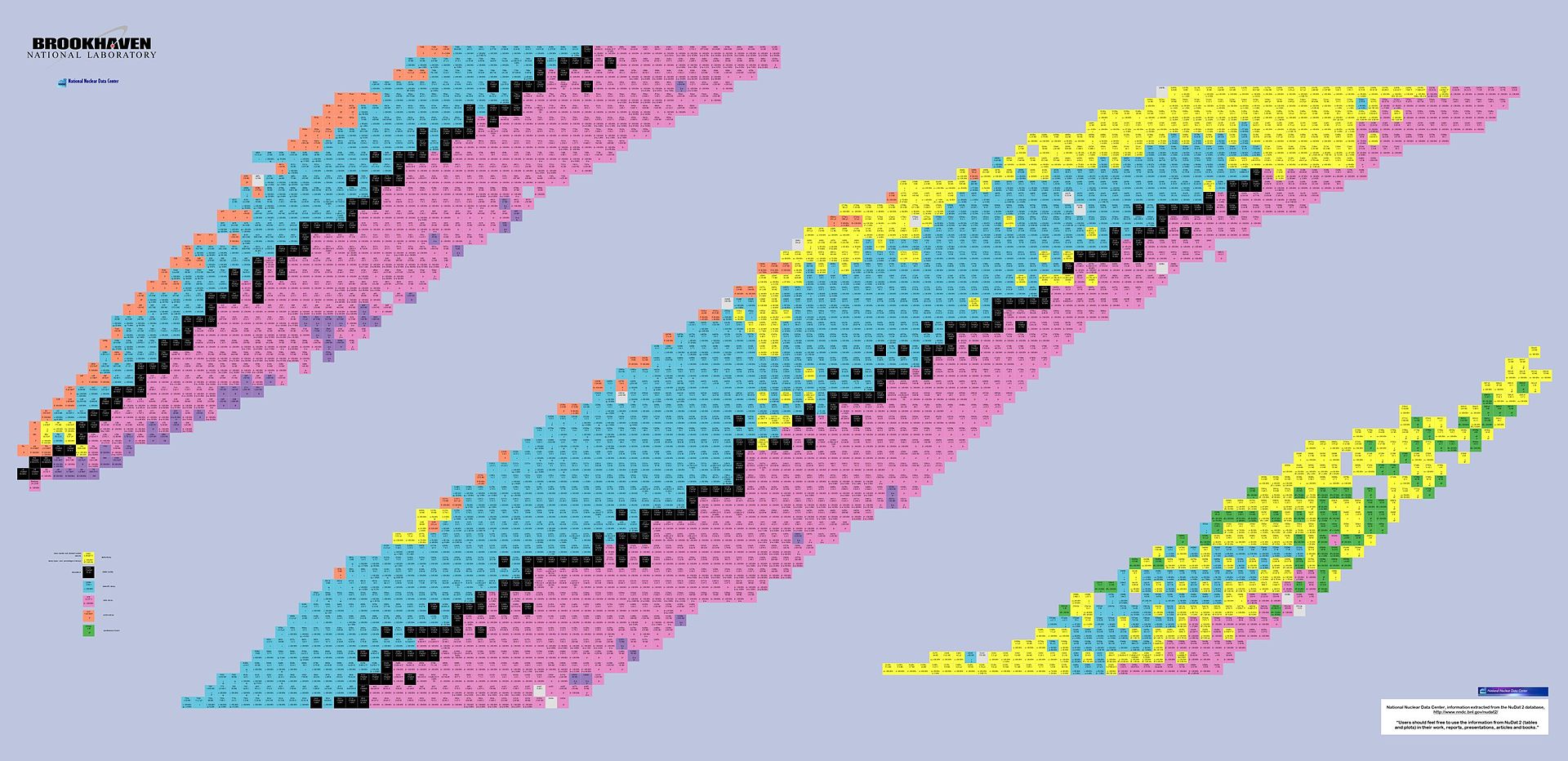
The stable atoms are shown in black, while those that undergo beta decay are shown in light blue.
Here's an interactive chart that offers more detail:
-
Answer:
A small introduction about three types of radio-activities see below.
$- Three main types.
@- Five additional types, for sake of completeness.Explanation:
See this to start with.
Different modes of beta decay are as follows. There is no change of mass number of the daughter nucleus. However the atomic number changes to one greater in case of
#beta^-# electron and changes to one less in case of#beta^+# positron decay.$ Electron emission,
#beta^-# decay. Here the nucleus emits an electron and an electron antineutrino#bar nu_e# . Example neutron changing into a proton.#""_0^1n -> ""_1^1p + ""^0e^− + bar nu_e# #""_55^137Cs -> ""_56^137Ba + ""^0e^− + bar nu_e# Positron emission,
#beta^+# decay. Here the nucleus emits a positron and an electron neutrino#nu# . Consider decay of a proton in the nucleus.#""_1^1p->""_0^1n+ ""^0e^+ +nu_e# #""_11^22Na ->""_10^22Ne +""^0e^++ nu_e# Electron capture, In this case the nucleus captures an orbiting electron and emits a neutrino. The daughter nucleus is unstable. Daughter nucleus has atomic number one less.
#""_11^22Na + ""^0e^− ->""_10^22Ne + nu_e# -.-.-.-.-.-.-.-.-.-.-.-.-.-..-.-.-.-..-.-.-
@ For sake of completeness. Following types of
#beta# decay are also included.Bound state beta decay, In this decay free neutron or nucleus beta decays to electron and antineutrino. The electron thus produced is not emitted but is captured by the atom to fill up one of its vacant electronic shells, the daughter nucleus is unstable.
Double beta decay, Similar to single electron case but some nucleus emits two electrons and two antineutrinos. Daughter nucleus has atomic number two more.
Double electron capture, some nucleus absorbs two orbital electrons and emits two neutrinos. The daughter nucleus is unstable and has atomic number two less.
Electron capture with positron emission, the nucleus absorbs one orbital electron, emits one positron and two neutrinos. Daughter nucleus has atomic number two less.
Double positron emission, A nucleus emits two positrons and two neutrinos. Daughter nucleus has atomic number two less.