In what way does increasing hybridization correlate with decreasing #s# character?
1 Answer
I think you mean this with respect to alkanes, alkenes, and alkynes. Consider ethane, ethene (ethylene), and ethyne (acetylene).
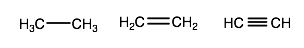
We know that bond lengths for comparable SIGMA bonds (e.g. the first bond in a
Well, from left to right, we have
Hybridization involves the mixing of more than one orbital shape to construct identical, totally symmetric orbitals. This literally means we have:
#sp^3 = s + p + p + p# #harr# #bb(25% s) + 75% p# #sp^2 = s + p + p" "" "# #harr# #bb(33% s) + 67% p# #sp = s + p" "" "" "" "# #harr# #bb(50% s) + 50% p#
We thus have an increase in
This decrease in bond length is due to the closeness of the
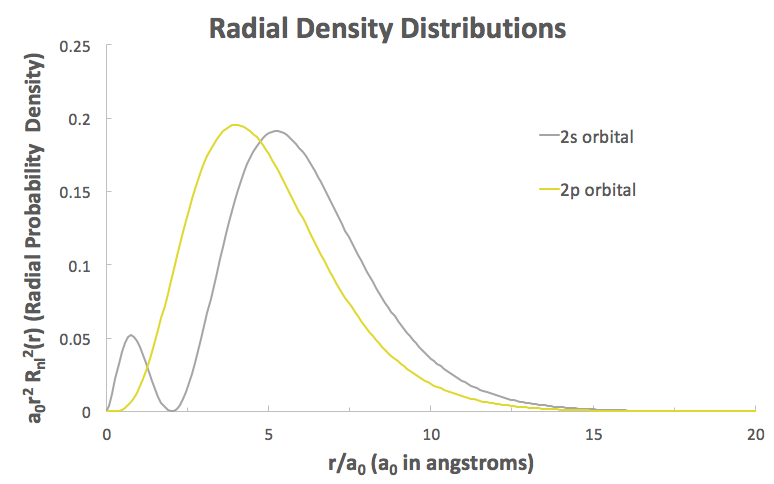
That little bump at the far left means that the
Hence, a hybrid orbital with more

