What were Rutherford's primary observations in his gold foil experiment, and how did he explain them? Describe how he modeled the atom.
1 Answer
DISCLAIMER: This is an old theory, and fails to hold true today, because its ideas imply that all atoms are unstable.
Rutherford's conclusion came while observing
The
 http://www.britannica.com/
http://www.britannica.com/
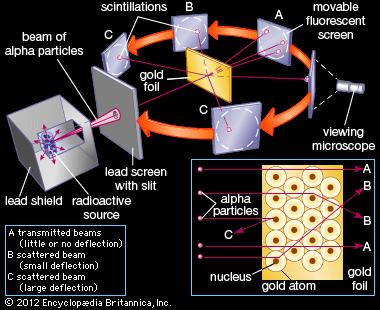
His two primary observations were:
- Most
α particles passed straight through the gold foil, which showed that atoms are mostly empty space. - Some of the
α particles were deflected at various angles, and sometimes even back at the radioactive source.
From the second observation, Rutherford concluded that it had to be due to a "central charge" that the interaction was enough to successfully deflect the
The interaction in more modern terms would be a nuclear repulsion. Specifically, of the helium and gold nuclei.
https://figures.boundless-cdn.com/
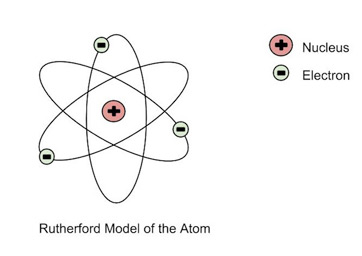
Afterwards, I guess it had something to do with his mindset back then, but he suggested that any negatively-charged particles around the nucleus balancing out the positive charge (probably) revolved around the nucleus.
He then probably approached it from a physics perspective, because he gave an explanation based on centrifugal force (an idea based on circular, orbiting motion) balancing out the electrostatic attraction of the electrons to the nucleus, perhaps because they were similar equations:
Fgrav=Gm1m2r2 (masses)
Fcoulomb=kq1q2r2=q1q24πε0r2 (charges)
(Note: centrifugal force is not a real force. It is just an inertial effect that gives the feel of an "apparent" force.)
This gave the Rutherford model: the "planetary model" of the atom.
 http://study.com/
http://study.com/
However, we know that electrons don't revolve around the nucleus, because if it were that simple, they would eventually dissipate their kinetic energy and spiral towards the nucleus, collapsing the atom. (That doesn't happen.)
The Rutherford atomic theory was superseded by:
- The Bohr model (consisting of quantized orbits; too grounded on certainty)
- ...and then, the modern-day Schrodinger model (consisting of probability densities and electron clouds; more grounded on uncertainty).

