Question #f635a
1 Answer
Here's what I got.
Explanation:
The first part of the question wants you to find a suitable set of quantum numbers for an electron located in a 4f-orbital.
As you know, four quantum numbers are used to describe the position and spin of an electron in an atom.
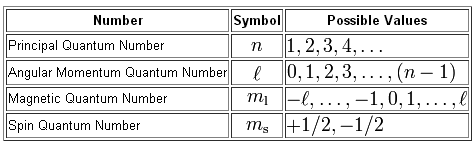
Now, the number that's added to the name of an orbital tells you the energy level on which the electron resides, i.e. the principal quantum number,
In your case, an electron located in a
Now, the angular momentum quantum number,
#l=0 -># the s-subshell#l=1 -># the p-subshell#l=2 -># the d-subshell#l=3 -># the f-subshell
Since your electron is located in the f-subshell, you will need
At this point, you can have any of the seven values for the magnetic quantum number,
#m_l = {-3,-2, -1, color(white)(-)0, +1, +2, +3}#
Likewise, the spin quantum number,
Therefore, your electron can have
#{(color(white)(a)n = color(red)(4)), (color(white)(a)l= 3), (m_l = {-3, -2, -1, color(white)(-)0, +1, +2, +3}), (m_s = {-1/2, + 1/2}) :}#
For the second part of the question, you must specify the subshell in which the electron is located.
For the first electron, you have
For the second electron, you have


