Question #32905
1 Answer
I can think of at least nine possible structures for tropic acid.
Explanation:
First Impressions
∴ The formula of tropic acid must be
The index of hydrogen deficiency is
Anytime I see
There is probably an
From the name tropic acid and a remaining
That leaves
Possible Structures
(a) If there is a monosubstituted phenyl
The possible structures are
(b) If there is a disubstituted phenyl
If there is a disubstituted phenyl, the possible structures are the ortho, meta, and para isomers of (1-hydroxyethyl)benzoic acid and (2-hydroxyethyl)benzoic acid.
We don’t have enough information to specify the structure of tropic acid.
The reactions
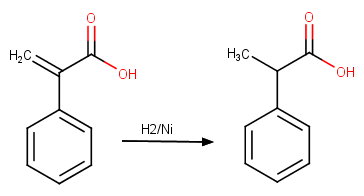
Assume the structure is
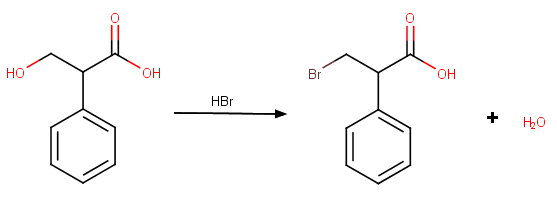
(a) Conversion of alcohol to alkyl bromide
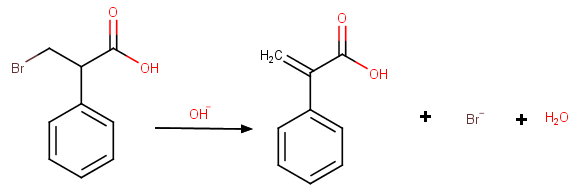
(b) Dehydrobromination to atropic acid
(c) Hydrogenation of alkene to form hydratropic acid