Question #12c06
1 Answer
Dec 21, 2016
Here's how I worked it out.
Explanation:
The cell is
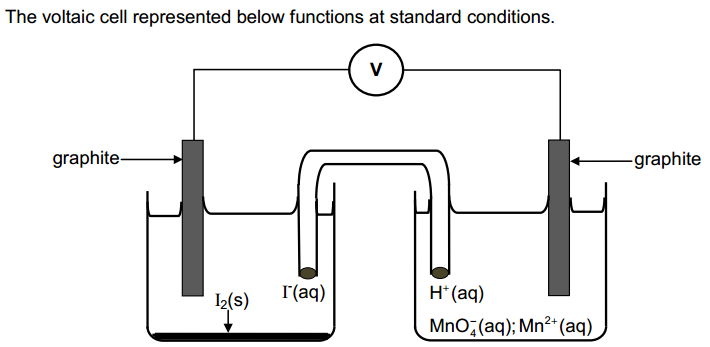
The cell reactions are:
Cell notation is a shorthand way of expressing a reaction in an electrochemical cell.
- The anode (oxidation) half-reaction is written on the left, and the cathode half-reaction goes on the right.
- The two half-reactions are separated by two bars or slashes representing a salt bridge.
- Individual solid, liquid, or aqueous phases within each half-cell are separated by a single bar.
- The reactants in each half cell are always written first, followed by the products.
- The state of each phase (usually s, l, g, or aq) is included after the species name.
Thus, the cell notation for this cell is

