Question #079e3
1 Answer
Explanation:
As you know, a total of four quantum numbers can be used to describe the location and spin of an electron inside an atom.
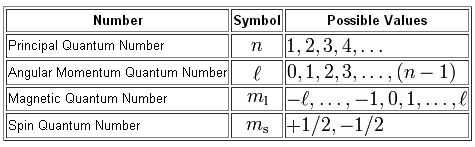
The problem wants you to find the value of the magnetic quantum number,
Now, the magnetic quantum number tells you the exact orbital in which the electron is located. As you can see, the magnetic quantum number depends on the angular momentum quantum number,
Simply put, every subshell will have a different number of orbitals as given be
#m_l = -l, ..., -1, 0, 1, ..., l#
A
#l =0 -># the s-subshell
As you can see, the magnetic quantum number can only take one possible value for
#m_l = 0 -># the s-orbital
This tells you that the s-subshell contains a single orbital, the s-oribtal. In this particular case, the

