Why can hydrogen not be the central atom in a compound?
1 Answer
Dec 24, 2016
Hydrogen atoms have only one electron and can form only one bond. In covalent bonding, a central atom must have enough available electrons to form at least two bonds.
Explanation:
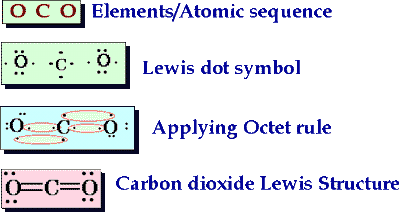
The diagram below shows the formation of a carbon dioxide molecule. The central atom is carbon, which has four electrons available for bonding. Each oxygen atom has two electrons available. Since the elements are nonmetals they will undergo covalent bonding. The double lines going from carbon to each oxygen atom represent double bonds.