Question #c0a10
1 Answer
Aug 21, 2016
When the protein shifts from the R-form to the T-form at pH 7.0, the histidine goes from its neutral to its ionic form.
Explanation:
The neutral and the ionic forms of the imidazole side-chain are shown below.
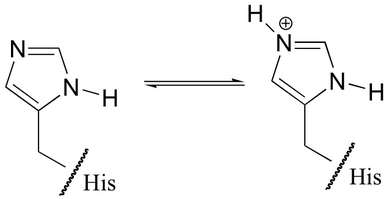
(From chem.libretexts.org)
There are three points to remember:
- If
#"pH = p"K_a# , the neutral and ionic forms are present in equal amounts. - If
#"pH < p"K_a# (i.e. more acidic), the ionic (protonated) form will predominate. - If
#"pH > p"K_a# (i.e. more basic), the neutral (non-protonated) form will predominate.
For the R-form,
∴ The R-form is ionic when pH <6.0 and neutral when pH > 6.0.
For the T-form,
∴ The T-form is ionic when pH < 8.1 and neutral when pH > 8.1.
At pH = 7.0, the R-form is neutral, but the T-form is ionic.
This corresponds to Statement 1.
Statement 2 is wrong, because the R-form is neutral at pH 7.0.
Statement 3 is wrong for the same reason.
Statement 4 is wrong, because the T-form is ionic at pH 7.0.
Statement 5 is wrong, for the same reason.

