Question #4341e
1 Answer
Sep 15, 2016
Here's how I do it.
Explanation:
Step 1. Draw the bond-line structure.
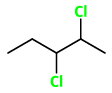
We see that there are two chiral carbons (those bearing the
The maximum number of stereoisomers is
There is no internal plane of symmetry, so the number of stereoisomers is
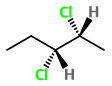
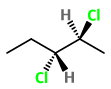
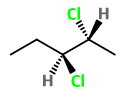
Step 2. Convert the structure to a wedge-dash formula.
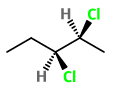
Here the
The four possible combinations are: wedge-wedge, dash-dash, wedge-dash, and dash-wedge.
Step 3. Draw the structures for the three remaining combinations..