When a gas is collected in an upturned graduated cylinder in water, to what will the pressure in the cylinder when the measurement is taken is equal?
a.the pressure of the gas PLUS Psaturated vapour pressure.
b.the pressure of the gas LESS Psaturated vapour pressure.
c.the pressure of the gas.
d.there is no relationship.
1 Answer
Well,
Explanation:
The given picture is the standard experimental setup.
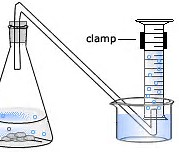 https://www.bestchoice.net.nz/chemistry/1219/p19281.htm
https://www.bestchoice.net.nz/chemistry/1219/p19281.htm
Clearly, the gas expands against atmospheric pressure. When the level of water in the graduated cylinder is equal to the level of water in the reservoir, the PRESSURE of the gas in the CYLINDER is equal to the ambient pressure LESS the
I again stress the level of water in the cylinder must match the level of water in the reservoir so that:
(sorry for all the SHOUTING! but I want to emphasize the approach.)

