What sort of meniscus does mercury form in a glass vessel?
2 Answers
Mercury metal forms a convex meniscus in glass vessels.
Explanation:
Metallic bonding clearly accounts for the convex meniscus observed in mercury metal in glass vessels. The metal is more attracted to itself than to the sides of the glass.
Of course if you use a PTFE container (and you can do so) you could form a convex meniscus with most liquids.
Mercury, which is liquid at room temperature, forms convex meniscus in glass barometers and thermometers.
Explanation:
The free surface of a liquid is called meniscus. It is flat, convex or concave, depending on the solid and liquid surface. It is dependent on intermolecular forces at the interface.
A convex meniscus is formed when the particles in the liquid have a stronger attraction to each other (force of cohesion) than to the material of the container (force of adhesion).
In the figure below forces acting on a molecule O of a liquid are shown
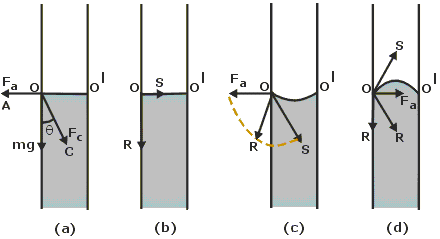
The forces are
- weight
#mg# of the molecule, acting vertically downward - force of adhesion
#F_a# acting along OA and - force of cohesion
#F_c# acting along OC, as shown in (a).
Resolving
-
If horizontal component of force of cohesion equals force of adhesion, remaining forces are weight of molecule and vertical component of force of cohesion of the molecule 'O'. Every point on the free liquid surface remains perpendicular. This results in the meniscus as flat as shown in (b).
-
If force of cohesion is less than the force of adhesion there is net horizontal force along OA outward. Consequently the resultant of horizontal and vertical forces, acts along OR in outward direction but is perpendicular to the liquid surface at O. Thus, resultant force on all liquid molecules on the free surface, acts perpendicular to the liquid surface as shown in (c), giving rise to a concave meniscus.
-
Similarly, if force of cohesion is more than the force of adhesion there is net horizontal inward force. Consequently the resultant of horizontal and vertical forces, acts along OR in inward direction but is perpendicular to the liquid surface at O. And resultant force on all liquid molecules on the free surface, acts perpendicular to the liquid surface as shown in (d), giving rise to a convex meniscus.

