Question #142a1
1 Answer
Sep 21, 2016
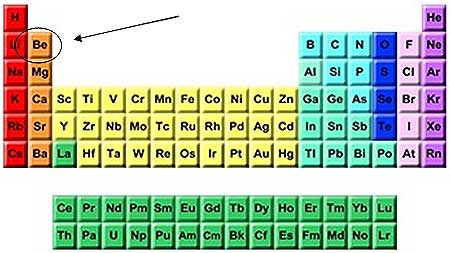
Beryllium.
Explanation:
The trick to remember here is that for main-group elements, i.e. elements located in groups 1, 2, and 13 through 18, the number of valence electrons is given by
- the group number for groups 1 and 2
- the units digit of the group number for groups 13 through 18
In order for an atom to form
This, of course, implies that this atom has two valence electrons. Consequently, this would place your atom in group 2 of the Periodic Table.
You now know that you're looking for the element located in period 2, group 2. A quick look in the periodic table will reveal that this element is beryllium,