Question #878a9
1 Answer
Manganese(II) chloride tetrahydrate.
Explanation:
You're dealing with a hydrate, which is a compound that consists of
- the anhydrous salt, which in this case is
#"MnCl"_2# - the water of crystallization, which in this case is
#4"H"_2"O"#
The first thing to do here is to focus on the anhydrous salt. Notice that the salt contains chloride anions, which as you know carry a
This implies that the charge on the manganese cation will be equal to
Because manganese is a transition metal, which implies that it can form cations of different charges, you must use a Roman numeral to name it.
In this case, the manganese cation carries a
#"Mn"^(2+) -># the manganese(II) cation
The anhydrous salt will be called
#"MnCl"_2 -># manganese(II) chloride
Now for the water of crystallization. Notice that each formula unit of this hydrate contains
- one formula unit of manganese(II) chloride,
#1 xx "MnCl"_2# - four molecules of water,
#4 xx "H"_2"O"#
At this point, you must turn to a Greek prefix.
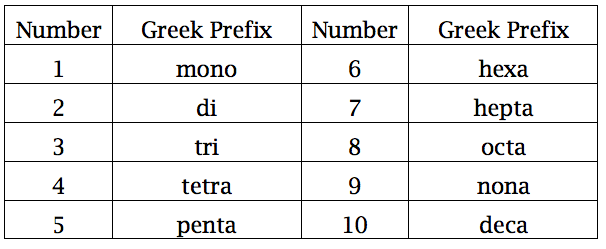
Since you have four water molecules per formula unit of hydrate, use the prefix tetra-.
The full name of the hydrate will thus be
#"MnCl"_2 * 4"H"_2"O" -># manganese(II) chloride tetrahydrate

