Why is methane nonpolar? Explain in terms of polarity within a bond, and overall polarity based on symmetry.
1 Answer
Polarity can either be within a bond, when one atom "hogs" the electrons more than the other, or in the overall molecule, when all the electrons tend to "group" to one side of the molecule but not the other.
A dipole simply refers to the two poles of a magnet. Similar to that, a chemical bond with a dipole suggests that one atom is partially negative and the other is partially positive. For example:
stackrel(delta^-)"C"-stackrel(delta^+)"H"
WITHIN A BOND
Polarity within a bond can be determined by looking at the electronegativity values (
"EN"_"H" = 2.1
"EN"_"C" = 2.5
So,
However, carbon does hog more of the electron density than hydrogen, so carbon is slightly negative, and we denote that as
OVERALL MOLECULE
Since each individual bond is not polar, we don't expect the molecule as a whole to be polar.
Let's pretend that each bond is polar enough to be polar though. Even then,
Drawing it like this gives the same molecule, but reveals more of the symmetry:
(all we did was rotate it counterclockwise, by
Since
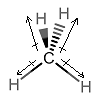
To imagine the effects of vectors, you can imagine gripping your two hands together and trying to pull in the exact opposite directions. That is the same as having one vector pulling to the left (your left hand), and one vector pulling to the right (your right hand) --- they cancel out, just like vectors do.
So, due to the properties of vectors:
- The bottom two arrows add downwards and cancel out horizontally, along the plane of the screen.
- The top two arrows add upwards and cancel out horizontally, perpendicular to the plane of the screen.
- Since they were all the same vertical magnitude to begin with (because they were the same bonds), the up and down arrow sums cancel out as well.
So, the net dipole moment of

