What would be the structure of #ICl_2^(-)# ion?
1 Answer
Dec 30, 2017
Is the ion not linear? Electronic geometry is trigonal bipyramidal.
Explanation:
There are
And so....
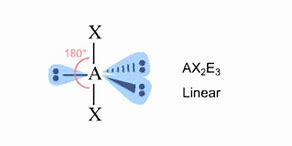
Because iodine is associated with EIGHT electrons, i.e. 3 lone pairs, and it is assigned 2 bonding electrons, it bears a FORMAL negative charge. The chlorine atoms are each associated with 7 electrons, and hence these centres are formally neutral.

