Question #fd6bc
1 Answer
Here's what's going on here.
Explanation:
In the video, Tyler says that
the oxygen and hydrogen pair can steal an electron from somewhere else
That basically means that in order to have a hydroxide anion,
In other words, the hydroxide anion is formed when the oxygen and hydrogen pair take an electron from elsewhere, usually from a metal atom.
A classic example would be sodium hydroxide,
When this oxygen and hydrogen group reacts with a sodium atom,
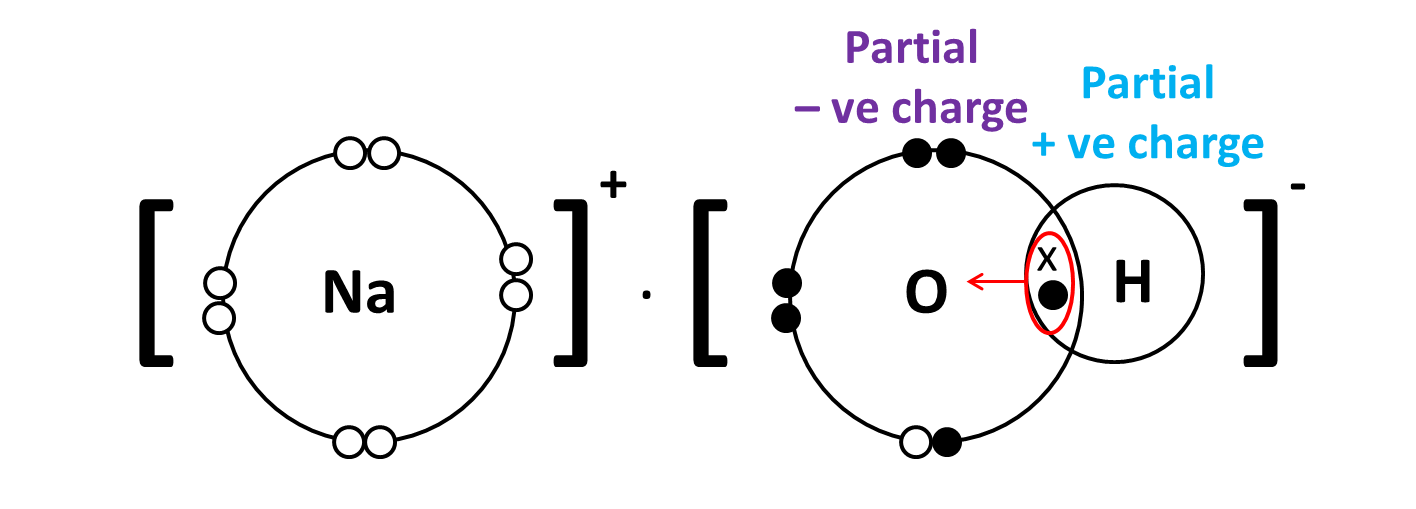
Another example to consider here is the auto-ionization of water, which produces hydronium cations,
#2"H"_ 2"O"_ ((l)) rightleftharpoons "H"_ 3"O"_ ((aq))^(+) + "OH"_ ((aq))^(-)#
Here one water molecule picks up a proton,
Long story short, the oxygen and hydrogen group that forms the hydroxide anion picks up an electron from another atom.
Tyler didn't mention this in that particular video because he wanted to focus exclusively on polyatomic ions and how their bonding works.

