How do we write the Lewis structure of the hypochlorite anion, ClO−?
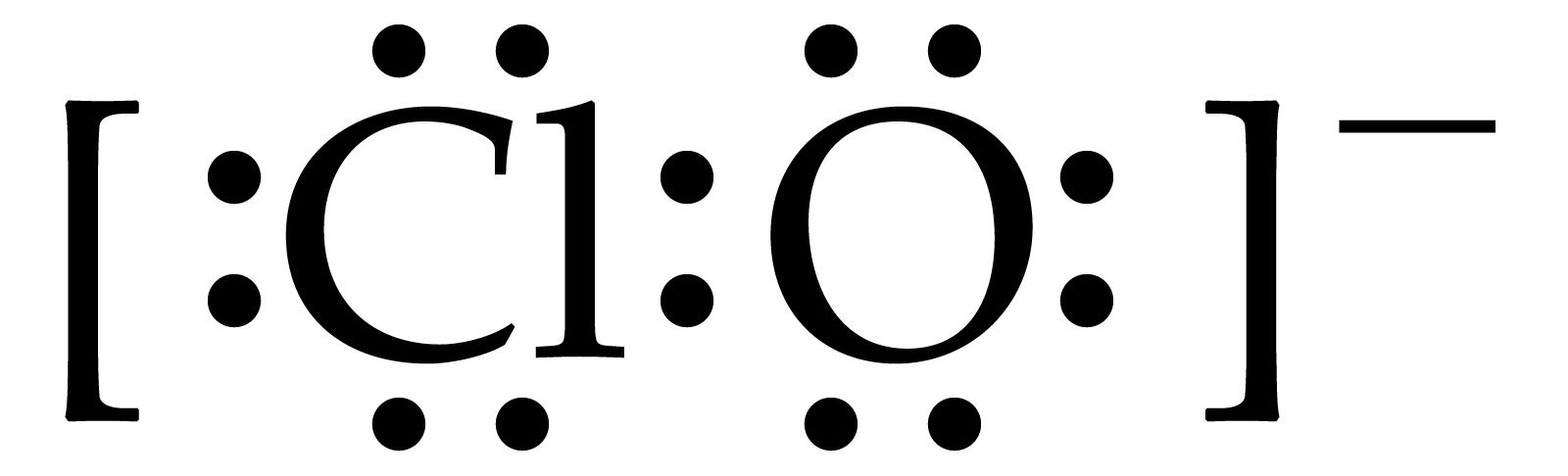 wps.prenhall.com
wps.prenhall.com
 wps.prenhall.com
wps.prenhall.com
1 Answer
Oct 8, 2016
These try to depict the electronic structure of
Explanation:
In fact the given diagram is highly misleading and ambiguous. The valence electrons around the chlorine atom were depicted. Why should only the chlorine-based electrons be? As written, the chlorine atom is neutral. A similar depiction should have been given around oxygen, and here a formal negative charge would be associated with the oxygen atom, as is required for the
For chlorate anion there are

