Question #34319
1 Answer
Explanation:
As you know, the only criterion that determines the spontaneity of a reaction is the Gibbs free energy change,
#color(blue)(bar(ul(|color(black)(DeltaG = DeltaH - T * DeltaS)color(white)(a/a)|)))#
Here
Now, in order for a reaction to be spontaneous at a given temperature, it must have
#DeltaG < 0#
This, of course, implies that a non-spontaneous reaction will have
#DeltaG > 0#
A positive Gibbs free energy change corresponds to
#DeltaH - T * DeltaS > 0#
This means that at low temperatures, you have
#DeltaH > T * DeltaS#
Now, this can be true for
This means that you need
#DeltaH - T * DeltaS < 0#
or
#DeltaH < T * DeltaS#
As you can see, this cannot be true if
#overbrace(DeltaH)^(color(blue)("negative")) > overbrace(T * DeltaS)^(color(blue)("even more negative")) -># non-spontaneous reaction
However, if
#overbrace(DeltaH)^(color(darkgreen)("positive")) < overbrace(T * DeltaS)^(color(darkgreen)("even more positive")) -># spontaneous reaction
Remember,
In general terms, you can have four possible scenarios when dealing with the Gibbs free energy change
#DeltaH<0# ,#DeltaS>0 -># spontaneous at any temperature#DeltaH>0# ,#DeltaS<0 -># non-spontaneous regardless of temperature#DeltaH>0# ,#DeltaS>0 -># spontaneous at a certain temperature range#DeltaH<0# ,#DeltaS<0 -># spontaneous at a certain temperature range
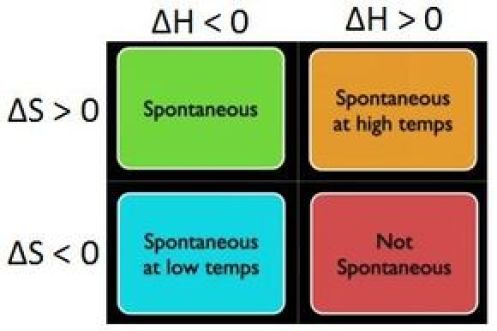
As you can see, reactions that have
In this particular case, the reaction is endothermic, since
A classic example would be the melting of ice, for which
#DeltaH > 0 -># you need to add heat to melt ice#DeltaS > 0 -># the entropy of the system is increasing because you're going from solid to liquid
However, the melting of ice is only spontaneous when

