Question #d8bb7
1 Answer
See explanation.
Explanation:
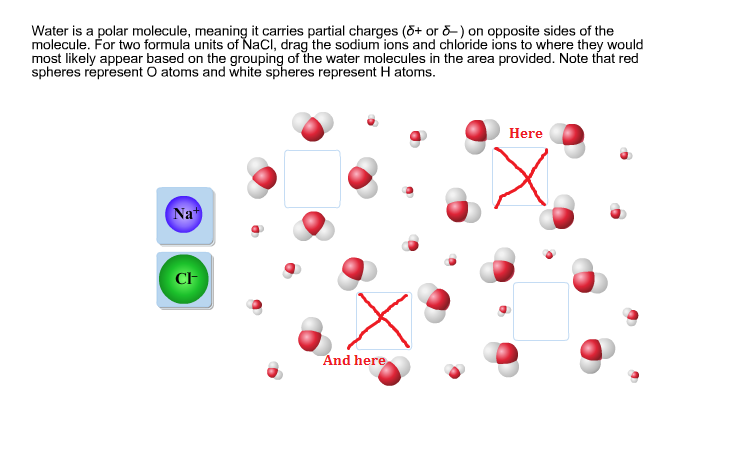
The reason why water is a polar molecule is because of the electronegativity difference between hydrogen and oxygen. Electronegativity is a fancy word for how much an atom wants electrons. The higher the electronegativity, the more an atom will pull in an electron.
Oxygen has a much higher electronegativity than hydrogen, so oxygen pulls in the electrons more. As a result, more electrons are around the oxygen atom, and oxygen has a partial negative charge. That means the electrons spend less time orbiting around the hydrogen atom - instead, they're hanging out with oxygen - so hydrogen has a partial positive charge.
The
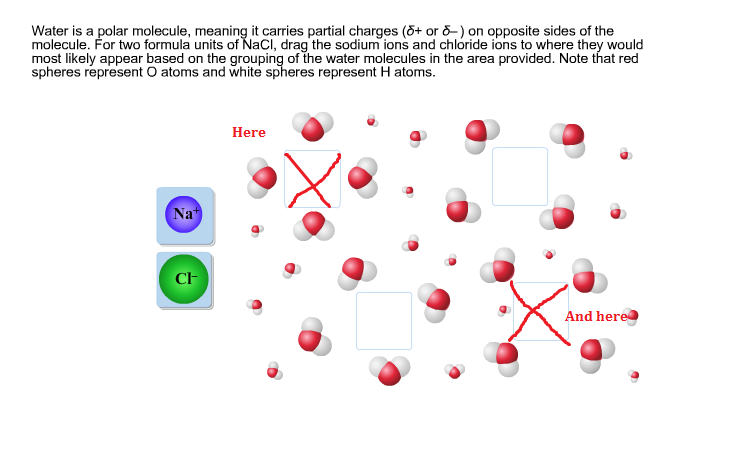
The