How does gas solubility change in the following scenarios, relative to the appropriate reference state?
#a)# The partial pressure of an anesthetic gas is increased.
#b)# #"O"_2# in the blood of a person who is in a hyperbaric chamber.
#c)# The pressure of a gas over a solvent is increased.
#d)# Air in the blood of a diver breathing compressed air that quickly surfaces.
#e)# The temperature is increased.
1 Answer
Well, maybe I can answer this for... other people, if not you.
Gas solubility is mathematically given, for ideal solutions, by Henry's law. For MasteringChemistry-related problems, this version is appropriate:
#s = k_HP_(gas)# where:
#s# is the solubility in#"mol/L"# of the gas.#k_H# is the Henry's law constant in#"M/atm"# .#P_(gas)# is the vapor pressure of the gas above the surface of the liquid.
Conceptually, solubility is simply the ability of the gas to stay in solution. Every gas particle has a certain escaping tendency, dependent on its speed.
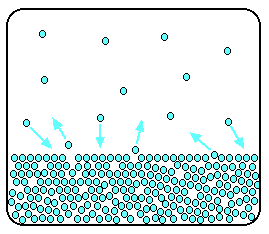
So, the faster the gas moves, the lower its solubility. And the gas solubility therefore depends...
- inversely on the temperature; i.e. higher temperature
#-># faster speed#-># lower solubility. - directly on the pressure above the solution, i.e. higher pressure
#-># the gas is pushed down into solution#-># the gas is more soluble.
Well, then its solubility increases, from the above reasoning. The escaping tendency decreased because the gas is pushed down in a closed vessel.
"Hyper" means heightened, and "baric" pertains to pressure... so a hyperbaric chamber is one in which the pressure is higher than usual.
Thus, the solubility increases for
#"O"_2# that one inhales, as one may experience in HBOT (hyperbaric oxygen therapy).
I think by now you know the answer to this one, if you followed the repetitive pattern in
#(a)# and#(b)# .
So... is the diver surfacing, or is the air surfacing? I'm guessing the diver, but who knows...
This one is a little tricky, but if you breathe compressed air while underwater, and you quickly surface to breathe regular air, the partial pressure of the air you breathe rapidly decreases.
The gas expands quickly, so the solubility of
#"O"_2# should decrease (not a good thing). This is why a diver should surface slowly, not quickly.
The gas thus moves faster, and escapes the solution more readily. Does the solubility now decrease or increase?

