Question #e9bc7
1 Answer
Explanation:
The thing to remember about nuclear equations is that they must conserve mass and charge.
In other words, the overall mass number and the overall atomic number must be equal on both sides of the equation.
In your case, you're dealing with a fusion reaction in which two helium-4 nuclides,
You will find
#"For He: " Z = 2#
#"For Be: " Z = 4#
Now, in isotope notation, the atomic number is added to the bottom-left and the mass number is added to the top-left of the chemical symbol.
You will thus have
#""_2^4"He" -># isotope notation for helium-4
#""_4^A"Be" -># isotope notation for the resulting isotope
Your goal now is to find the value of
#""_color(blue)(2)^color(orange)(4)"He" + ""_color(blue)(2)^color(orange)(4)"He" -> ""_color(blue)(4)^color(orange)(A)"Be"#
Notice that you have
#color(blue)(2) + color(blue)(2) = color(blue)(4) -># charge is conserved
You also need to have
#color(orange)(4) + color(orange)(4) = color(orange)(A) -># mass must be conserved as well
The value of
#4 + 4 = A implies A = 8#
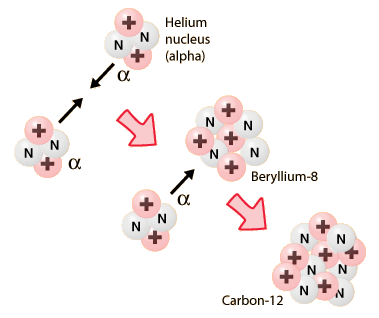
Therefore, the resulting isotope is beryllium-8 and the balanced nuclear equation that describes this fusion reaction looks like this
#""_2^4"He" + ""_2^4"He" -> ""_4^8"Be"#
As a side note, notice what happens when the beryllium-8 isotope fuses with another alpha particle
#""_ 4^8"Be" + ""_ 2^4"He" -> ""_ (color(white)(1)6)^12"C"#
The resulting nuclide is carbon-12, the most abundant isotope of carbon.